Tartrate-resistant acid phosphatase 5 promotes pulmonary fibrosis by modulating β-catenin signaling
- PMID: 35013220
- PMCID: PMC8748833
- DOI: 10.1038/s41467-021-27684-9
Tartrate-resistant acid phosphatase 5 promotes pulmonary fibrosis by modulating β-catenin signaling
Abstract
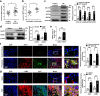
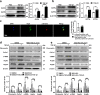
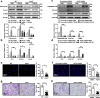
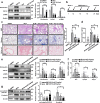
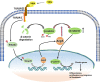
Idiopathic pulmonary fibrosis (IPF) is a fatal interstitial lung disease with limited therapeutic options. Tartrate-resistant acid phosphatase 5 (ACP5) performs a variety of functions. However, its role in IPF remains unclear. Here, we demonstrate that the levels of ACP5 are increased in IPF patient samples and mice with bleomycin (BLM)-induced pulmonary fibrosis. In particular, higher levels of ACP5 are present in the sera of IPF patients with a diffusing capacity of the lungs for carbonmonoxide (DLCO) less than 40% of the predicted value. Additionally, Acp5 deficiency protects mice from BLM-induced lung injury and fibrosis coupled with a significant reduction of fibroblast differentiation and proliferation. Mechanistic studies reveal that Acp5 is upregulated by transforming growth factor-β1 (TGF-β1) in a TGF-β receptor 1 (TGFβR1)/Smad family member 3 (Smad3)-dependent manner, after which Acp5 dephosphorylates p-β-catenin at serine 33 and threonine 41, inhibiting the degradation of β-catenin and subsequently enhancing β-catenin signaling in the nucleus, which promotes the differentiation, proliferation and migration of fibroblast. More importantly, the treatment of mice with Acp5 siRNA-loaded liposomes or Acp5 inhibitor reverses established lung fibrosis. In conclusions, Acp5 is involved in the initiation and progression of pulmonary fibrosis and strategies aimed at silencing or suppressing Acp5 could be considered as potential therapeutic approaches against pulmonary fibrosis.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

