Chirality transmission in macromolecular domains
- PMID: 35013247
- PMCID: PMC8748818
- DOI: 10.1038/s41467-021-27708-4
Chirality transmission in macromolecular domains
Abstract
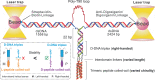
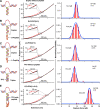
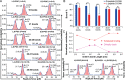
Chiral communications exist in secondary structures of foldamers and copolymers via a network of noncovalent interactions within effective intermolecular force (IMF) range. It is not known whether long-range chiral communication exists between macromolecular tertiary structures such as peptide coiled-coils beyond the IMF distance. Harnessing the high sensitivity of single-molecule force spectroscopy, we investigate the chiral interaction between covalently linked DNA duplexes and peptide coiled-coils by evaluating the binding of a diastereomeric pair of three DNA-peptide conjugates. We find that right-handed DNA triple helices well accommodate peptide triple coiled-coils of the same handedness, but not with the left-handed coiled-coil stereoisomers. This chiral communication is effective in a range (<4.5 nm) far beyond canonical IMF distance. Small-angle X-ray scattering and molecular dynamics simulation indicate that the interdomain linkers are tightly packed via hydrophobic interactions, which likely sustains the chirality transmission between DNA and peptide domains. Our findings establish that long-range chiral transmission occurs in tertiary macromolecular domains, explaining the presence of homochiral pairing of superhelices in proteins.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Structural and biochemical characterizations of an intramolecular tandem coiled coil protein.Biochem Biophys Res Commun. 2014 Dec 12;455(3-4):339-46. doi: 10.1016/j.bbrc.2014.11.013. Epub 2014 Nov 15. Biochem Biophys Res Commun. 2014. PMID: 25446089
-
Chirality of coiled coils: elasticity matters.Phys Rev Lett. 2008 Jan 25;100(3):038105. doi: 10.1103/PhysRevLett.100.038105. Epub 2008 Jan 25. Phys Rev Lett. 2008. PMID: 18233043
-
Homochirality Originates from the Handedness of Helices.J Phys Chem Lett. 2020 Oct 15;11(20):8690-8696. doi: 10.1021/acs.jpclett.0c02144. Epub 2020 Sep 29. J Phys Chem Lett. 2020. PMID: 32969653
-
Pharmacological interference with protein-protein interactions mediated by coiled-coil motifs.Handb Exp Pharmacol. 2008;(186):461-82. doi: 10.1007/978-3-540-72843-6_19. Handb Exp Pharmacol. 2008. PMID: 18491064 Review.
-
Chirality in peptide-based materials: From chirality effects to potential applications.Chirality. 2021 Oct;33(10):618-642. doi: 10.1002/chir.23344. Epub 2021 Aug 2. Chirality. 2021. PMID: 34342057 Review.
Cited by
-
Nanoparticles exhibiting virus-mimic surface topology for enhanced oral delivery.Nat Commun. 2023 Nov 24;14(1):7694. doi: 10.1038/s41467-023-43465-y. Nat Commun. 2023. PMID: 38001086 Free PMC article.
-
Supramolecular "sergeants": in situ and multi-level induction of chirality in helical assemblies of triarylamine trisamide monomers.Chem Sci. 2025 Jul 10. doi: 10.1039/d5sc02159f. Online ahead of print. Chem Sci. 2025. PMID: 40687690 Free PMC article.
-
Uncovering the chiral bias of meteoritic isovaline through asymmetric photochemistry.Nat Commun. 2023 Jun 8;14(1):3381. doi: 10.1038/s41467-023-39177-y. Nat Commun. 2023. PMID: 37291172 Free PMC article.
-
Chemo-mechanical forces modulate the topology dynamics of mesoscale DNA assemblies.Nat Commun. 2023 Oct 13;14(1):6459. doi: 10.1038/s41467-023-41604-z. Nat Commun. 2023. PMID: 37833326 Free PMC article.
-
Construction of chiral capillary electrochromatography microsystems based on Aspergillus sp. CM96.Mikrochim Acta. 2023 Aug 19;190(9):357. doi: 10.1007/s00604-023-05926-5. Mikrochim Acta. 2023. PMID: 37597027
References
-
- Krautwald S, Carreira EM. Stereodivergence in asymmetric catalysis. J. Am. Chem. Soc. 2017;139:5627–5639. - PubMed
-
- Proctor RSJ, Colgan AC, Phipps RJ. Exploiting attractive non-covalent interactions for the enantioselective catalysis of reactions involving radical intermediates. Nat. Chem. 2020;12:990–1004. - PubMed
-
- Jain V, et al. Chiral cooperativity in helical polymers. Isr. J. Chem. 2011;51:1067–1074.
-
- Suginome M, Yamamoto T, Nagata Y. Poly(quinoxaline-2,3-diyl)s: a fascinating helical macromolecular scaffold for new chiral functions. J. Synth. Org. Chem. Jpn. 2015;73:87–101.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

