Synthetic prodrug design enables biocatalytic activation in mice to elicit tumor growth suppression
- PMID: 35013295
- PMCID: PMC8748823
- DOI: 10.1038/s41467-021-27804-5
Synthetic prodrug design enables biocatalytic activation in mice to elicit tumor growth suppression
Abstract
Considering the intrinsic toxicities of transition metals, their incorporation into drug therapies must operate at minimal amounts while ensuring adequate catalytic activity within complex biological systems. As a way to address this issue, this study investigates the design of synthetic prodrugs that are not only tuned to be harmless, but can be robustly transformed in vivo to reach therapeutically relevant levels. To accomplish this, retrosynthetic prodrug design highlights the potential of naphthylcombretastatin-based prodrugs, which form highly active cytostatic agents via sequential ring-closing metathesis and aromatization. Structural adjustments will also be done to improve aspects related to catalytic reactivity, intrinsic bioactivity, and hydrolytic stability. The developed prodrug therapy is found to possess excellent anticancer activities in cell-based assays. Furthermore, in vivo activation by intravenously administered glycosylated artificial metalloenzymes can also induce significant reduction of implanted tumor growth in mice.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
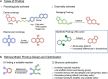
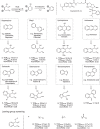




References
-
- Choi-Sledeski, Y. M. & Wermuth, C. G. Designing prodrugs and bioprecursors. in The Practice of Medicinal Chemistry: Fourth Edition 657–696 10.1016/B978-0-12-417205-0.00028-6 (Elsevier Inc., 2015).
-
- Tu J, Xu M, Franzini RM. Dissociative bioorthogonal reactions. ChemBioChem. 2019;20:1615–1627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

