Characterization of cisplatin-loaded chitosan nanoparticles and rituximab-linked surfaces as target-specific injectable nano-formulations for combating cancer
- PMID: 35013493
- PMCID: PMC8748743
- DOI: 10.1038/s41598-021-04427-w
Characterization of cisplatin-loaded chitosan nanoparticles and rituximab-linked surfaces as target-specific injectable nano-formulations for combating cancer
Abstract
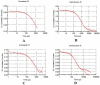
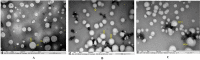
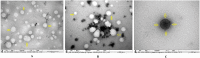
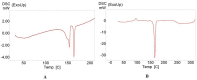
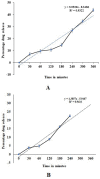
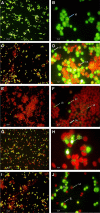
The present study was carried out to develop cisplatin-loaded chitosan nanoparticles (CCNP) and cisplatin-loaded chitosan nanoparticle surface linked to rituximab (mAbCCNP) as targeted delivery formulations. The two formulations (CCNP and mAbCCNP) exhibited significant physicochemical properties. The zetapotential (ZP) values of CCNP and mAbCCNP were 30.50 ± 5.64 and 26.90 ± 9.09 mV, respectively; while their particle sizes were 308.10 ± 1.10 and 349.40 ± 3.20 z.d.nm, respectively. The poly dispersity index (PDI) of CCNP was 0.257 ± 0.030 (66.6% PDI), while that of mAbCCNP was 0.444 ± 0.007 (57.60% PDI). Differential scanning calorimetry (DSC) revealed that CCNP had endothermic peaks at temperatures ranging from 135.50 to 157.69 °C. A sharp exothermic peak was observed at 95.79 °C, and an endothermic peak was observed at 166.60 °C. The XRD study on CCNP and mAbCCNP revealed distinct peaks at 2θ. Four peaks at 35.38°, 37.47°, 49.29°, and 59.94° corresponded to CCNP, while three distinct peaks at 36.6°, 49.12°, and 55.08° corresponded to mAbCCNP. The in vitro release of cisplatin from nanoparticles followed zero order kinetics in both CCNP and mAbCCNP. The profile for CCNP showed 43.80% release of cisplatin in 6 h (R2 = 0.9322), indicating linearity of release with minimal deviation. However, the release profile of mAbCCNP showed 22.52% release in 4 h (R2 = 0.9416), indicating linearity with sustained release. In vitro cytotoxicity studies on MCF-7 ATCC human breast cancer cell line showed that CCNP exerted good cytotoxicity, with IC50 of 4.085 ± 0.065 µg/mL. However, mAbCCNP did not elicit any cytotoxic effect. At a dose of 4.00 µg/mL cisplatin induced early apoptosis and late apoptosis, chromatin condensation, while it produced secondary necrosis at a dose of 8.00 µg/mL. Potential delivery system for cisplatin CCNP and mAbCCNP were successfully formulated. The results indicated that CCNP was a more successful formulation than mAbCCNP due to lack of specificity of rituximab against MCF-7 ATCC human breast cancer cells.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Raju, V., Chandrababu, R., Ramar, T. Chapter 12—Targeted Nanotherapeutics Based on Cancer Biomarkers, Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics, 229–244 10.1016/B978-0-323-52725-5.00012-5 (Elsevier Publications, 2017).
-
- Safhi, M.M., et al. Chapter 8—Nanoparticle System for Anticancer Drug Delivery: Targeting to Overcome Multidrug Resistance, Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics. 159–169 10.1016/B978-0-323-52725-5.00008-3 (Elsevier Publications, 2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

