Novel biallelic variants expand the SLC5A6-related phenotypic spectrum
- PMID: 35013551
- PMCID: PMC8747999
- DOI: 10.1038/s41431-021-01033-2
Novel biallelic variants expand the SLC5A6-related phenotypic spectrum
Abstract
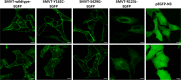
The sodium (Na+):multivitamin transporter (SMVT), encoded by SLC5A6, belongs to the sodium:solute symporter family and is required for the Na+-dependent uptake of biotin (vitamin B7), pantothenic acid (vitamin B5), the vitamin-like substance α-lipoic acid, and iodide. Compound heterozygous SLC5A6 variants have been reported in individuals with variable multisystemic disorder, including failure to thrive, developmental delay, seizures, cerebral palsy, brain atrophy, gastrointestinal problems, immunodeficiency, and/or osteopenia. We expand the phenotypic spectrum associated with biallelic SLC5A6 variants affecting function by reporting five individuals from three families with motor neuropathies. We identified the homozygous variant c.1285 A > G [p.(Ser429Gly)] in three affected siblings and a simplex patient and the maternally inherited c.280 C > T [p.(Arg94*)] variant and the paternally inherited c.485 A > G [p.(Tyr162Cys)] variant in the simplex patient of the third family. Both missense variants were predicted to affect function by in silico tools. 3D homology modeling of the human SMVT revealed 13 transmembrane helices (TMs) and Tyr162 and Ser429 to be located at the cytoplasmic facing region of TM4 and within TM11, respectively. The SLC5A6 missense variants p.(Tyr162Cys) and p.(Ser429Gly) did not affect plasma membrane localization of the ectopically expressed multivitamin transporter suggesting reduced but not abolished function, such as lower catalytic activity. Targeted therapeutic intervention yielded clinical improvement in four of the five patients. Early molecular diagnosis by exome sequencing is essential for timely replacement therapy in affected individuals.
© 2022. The Author(s).
Conflict of interest statement
LR is an employee of GeneDx, Inc and PR is an employee and KMG is a director of Suma Genomics Pvt. Ltd. All other authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

