The ubiquitin-dependent ATPase p97 removes cytotoxic trapped PARP1 from chromatin
- PMID: 35013556
- PMCID: PMC8760077
- DOI: 10.1038/s41556-021-00807-6
The ubiquitin-dependent ATPase p97 removes cytotoxic trapped PARP1 from chromatin
Abstract
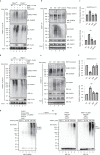
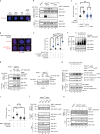
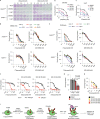
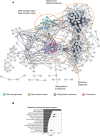
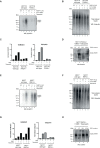
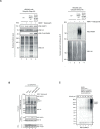
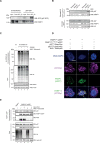
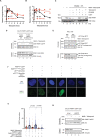
Poly (ADP-ribose) polymerase (PARP) inhibitors elicit antitumour activity in homologous recombination-defective cancers by trapping PARP1 in a chromatin-bound state. How cells process trapped PARP1 remains unclear. Using wild-type and a trapping-deficient PARP1 mutant combined with rapid immunoprecipitation mass spectrometry of endogenous proteins and Apex2 proximity labelling, we delineated mass spectrometry-based interactomes of trapped and non-trapped PARP1. These analyses identified an interaction between trapped PARP1 and the ubiquitin-regulated p97 ATPase/segregase. We found that following trapping, PARP1 is SUMOylated by PIAS4 and subsequently ubiquitylated by the SUMO-targeted E3 ubiquitin ligase RNF4, events that promote recruitment of p97 and removal of trapped PARP1 from chromatin. Small-molecule p97-complex inhibitors, including a metabolite of the clinically used drug disulfiram (CuET), prolonged PARP1 trapping and enhanced PARP inhibitor-induced cytotoxicity in homologous recombination-defective tumour cells and patient-derived tumour organoids. Together, these results suggest that p97 ATPase plays a key role in the processing of trapped PARP1 and the response of tumour cells to PARP inhibitors.
© 2022. The Author(s).
Conflict of interest statement
T.T.T. is a co-founder of Hysplex, LLC, with interests in PARPi development. C.J.L. makes the following disclosures: is/has been a consultant for AstraZeneca, Merck KGaA, Artios, Syncona, Sun Pharma, Gerson Lehrman Group, Vertex, Tango, 3rd Rock, Ono Pharma, Dark Blue Therapeutics, Horizon Discovery and Abingworth; has received grant/research support from AstraZeneca, Artios and Merck KGaA; is a stockholder in Tango and Ovibio; and stands to gain from the use of PARP and other DNA-repair inhibitors as part of the Institute of Cancer Research ‘rewards to inventors’ scheme. A.N.J.T. is/has been a consultant for AstraZeneca, Merck KGaA, Artios, Pfizer, Vertex, GE Healthcare, Inbiomotion and MD Anderson Cancer Centre; has received grant/research support from AstraZeneca, Myriad, Medivation and Merck KGaA; is a stockholder in Inbiomotion; and stands to gain from the use of PARPi as part of the Institute of Cancer Research ‘rewards to inventors’ scheme. The remaining authors declare no competing interests.
Figures





Comment in
-
Unsprung traps keep PARP inhibitors effective.Nat Cell Biol. 2022 Jan;24(1):2-4. doi: 10.1038/s41556-021-00819-2. Nat Cell Biol. 2022. PMID: 35013555 No abstract available.
-
Releasing the trap: How the segregase p97 extracts PARP1 from chromatin.Mol Cell. 2022 Mar 3;82(5):889-890. doi: 10.1016/j.molcel.2022.02.012. Mol Cell. 2022. PMID: 35245455
References
-
- Gogola E, et al. Selective loss of PARG restores PARylation and counteracts PARP inhibitor-mediated synthetic lethality. Cancer Cell. 2018;33:1078–1093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

