Dynamic diel proteome and daytime nitrogenase activity supports buoyancy in the cyanobacterium Trichodesmium
- PMID: 35013592
- PMCID: PMC10288448
- DOI: 10.1038/s41564-021-01028-1
Dynamic diel proteome and daytime nitrogenase activity supports buoyancy in the cyanobacterium Trichodesmium
Abstract
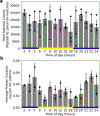
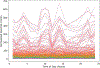
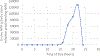
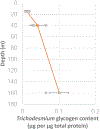
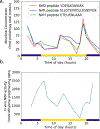
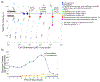
Cyanobacteria of the genus Trichodesmium provide about 80 Tg of fixed nitrogen to the surface ocean per year and contribute to marine biogeochemistry, including the sequestration of carbon dioxide. Trichodesmium fixes nitrogen in the daylight, despite the incompatibility of the nitrogenase enzyme with oxygen produced during photosynthesis. While the mechanisms protecting nitrogenase remain unclear, all proposed strategies require considerable resource investment. Here we identify a crucial benefit of daytime nitrogen fixation in Trichodesmium spp. that may counteract these costs. We analysed diel proteomes of cultured and field populations of Trichodesmium in comparison with the marine diazotroph Crocosphaera watsonii WH8501, which fixes nitrogen at night. Trichodesmium's proteome is extraordinarily dynamic and demonstrates simultaneous photosynthesis and nitrogen fixation, resulting in balanced particulate organic carbon and particulate organic nitrogen production. Unlike Crocosphaera, which produces large quantities of glycogen as an energy store for nitrogenase, proteomic evidence is consistent with the idea that Trichodesmium reduces the need to produce glycogen by supplying energy directly to nitrogenase via soluble ferredoxin charged by the photosynthesis protein PsaC. This minimizes ballast associated with glycogen, reducing cell density and decreasing sinking velocity, thus supporting Trichodesmium's niche as a buoyant, high-light-adapted colony forming cyanobacterium. To occupy its niche of simultaneous nitrogen fixation and photosynthesis, Trichodesmium appears to be a conspicuous consumer of iron, and has therefore developed unique iron-acquisition strategies, including the use of iron-rich dust. Particle capture by buoyant Trichodesmium colonies may increase the residence time and degradation of mineral iron in the euphotic zone. These findings describe how cellular biochemistry defines and reinforces the ecological and biogeochemical function of these keystone marine diazotrophs.
© 2022. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures







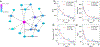
Comment in
-
Iron keeps Trichodesmium afloat.Nat Rev Microbiol. 2022 Mar;20(3):126. doi: 10.1038/s41579-022-00690-4. Nat Rev Microbiol. 2022. PMID: 35027702 No abstract available.
References
-
- Zehr JP Capone DG Changing perspectives in marine nitrogen fixation. Science 9514, 729 (2020). - PubMed
-
- Karl D et al. Dinitrogen fixation in the world’s oceans. Biogeochemistry 57–58, 47–98 (2002).
-
- Dugdale R & Wilkerson F Nutrient Limitation of New Production in the Sea. in Primary Productivity and Biogeochemical Cycles in the Sea (eds. Falkowski PG, Woodhead AD & Vivirito K) 107–122 (Springer; US, 1992). doi: 10.1007/978-1-4899-0762-2_7. - DOI
-
- Carpenter EJ & Capone DG Nitrogen Fixation in the Marine Environment. in Nitrogen in the Marine Environment (Elsevier, 2008). doi: 10.1016/B978-0-12-372522-6.00004-9. - DOI
-
- Gruber N, Sarmiento JL Global patterns of marine nitrogen fixation and denitrification. Global Biogeochem. Cycles 11, 23–266 (1997).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

