Pharmacological activation of the Sonic hedgehog pathway with a Smoothened small molecule agonist ameliorates the severity of alcohol-induced morphological and behavioral birth defects in a zebrafish model of fetal alcohol spectrum disorder
- PMID: 35014067
- PMCID: PMC9271529
- DOI: 10.1002/jnr.25008
Pharmacological activation of the Sonic hedgehog pathway with a Smoothened small molecule agonist ameliorates the severity of alcohol-induced morphological and behavioral birth defects in a zebrafish model of fetal alcohol spectrum disorder
Abstract
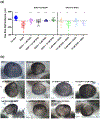
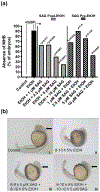
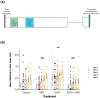
Ethanol exposure during the early stages of embryonic development can lead to a range of morphological and behavioral differences termed fetal alcohol spectrum disorders (FASDs). In a zebrafish model, we have shown that acute ethanol exposure at 8-10 hr postfertilization (hpf), a critical time of development, produces birth defects similar to those clinically characterized in FASD. Dysregulation of the Sonic hedgehog (Shh) pathway has been implicated as a molecular basis for many of the birth defects caused by prenatal alcohol exposure. We observed in zebrafish embryos that shh expression was significantly decreased by ethanol exposure at 8-10 hpf, while smo expression was much less affected. Treatment of zebrafish embryos with SAG or purmorphamine, small molecule Smoothened agonists that activate Shh signaling, ameliorated the severity of ethanol-induced developmental malformations including altered eye size and midline brain development. Furthermore, this rescue effect of Smo activation was dose dependent and occurred primarily when treatment was given after ethanol exposure. Markers of Shh signaling (gli1/2) and eye development (pax6a) were restored in embryos treated with SAG post-ethanol exposure. Since embryonic ethanol exposure has been shown to produce later-life neurobehavioral impairments, juvenile zebrafish were examined in the novel tank diving test. Our results further demonstrated that in zebrafish embryos exposed to ethanol, SAG treatment was able to mitigate long-term neurodevelopmental impairments related to anxiety and risk-taking behavior. Our results indicate that pharmacological activation of the Shh pathway at specific developmental timing markedly diminishes the severity of alcohol-induced birth defects.
Keywords: FASD; RRID:CVCL_0190; RRID:SCR_000441; RRID:SCR_002798; SAG; SMO; Shh; behavior; zebrafish.
© 2022 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST
The authors declare no competing financial interests.
Figures



References
-
- Albert S, Müller F, Fischer N, Biellmann D, Neumann C, Blader P, & Strähle U (2003). Cyclops-independent floor plate differentiation in zebrafish embryos. Developmental dynamics: an official publication of the American Association of Anatomists, 226(1), 59–66. - PubMed
-
- Aoto K, Shikata Y, Higashiyama D, Shiota K, & Motoyama J (2008). Fetal ethanol exposure activates protein kinase A and impairs Shh expression in prechordal mesendoderm cells in the pathogenesis of holoprosencephaly. Birth Defects Research Part A: Clinical and Molecular Teratology, 82(4), 224–231. - PubMed
-
- Arenzana F, Carvan MJ III, Aijon J, Sanchez-Gonzalez R, Arevalo R, & Porteros A (2006). Teratogenic effects of ethanol exposure on zebrafish visual system development. Neurotoxicology and teratology, 28(3), 342–348. - PubMed
-
- Arsić D, Beasley SW, & Sullivan MJ (2007). Switched-on Sonic hedgehog: A gene whose activity extends beyond fetal development–to oncogenesis. Journal of paediatrics and child health, 43(6), 421–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

