B-value and empirical equivalence bound: A new procedure of hypothesis testing
- PMID: 35014082
- PMCID: PMC8881334
- DOI: 10.1002/sim.9298
B-value and empirical equivalence bound: A new procedure of hypothesis testing
Abstract
In this study, we propose a two-stage procedure for hypothesis testing, where the first stage is conventional hypothesis testing and the second is an equivalence testing procedure using an introduced empirical equivalence bound (EEB). In 2016, the American Statistical Association released a policy statement on P-values to clarify the proper use and interpretation in response to the criticism of reproducibility and replicability in scientific findings. A recent solution to improve reproducibility and transparency in statistical hypothesis testing is to integrate P-values (or confidence intervals) with practical or scientific significance. Similar ideas have been proposed via the equivalence test, where the goal is to infer equality under a presumption (null) of inequality of parameters. However, the definition of scientific significance/equivalence can sometimes be ill-justified and subjective. To circumvent this drawback, we introduce the B-value and the EEB, which are both estimated from the data. Performing a second-stage equivalence test, our procedure offers an opportunity to improve the reproducibility of findings across studies.
Keywords: empirical equivalence bound; equivalence test; hypothesis testing.
© 2022 John Wiley & Sons, Ltd.
Figures


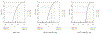
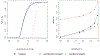
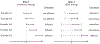


References
-
- Hoenig JM, Heisey DM. The abuse of power: the pervasive fallacy of power calculations for data analysis. The American Statistician 2001; 55(1): 19–24.
-
- Westlake WJ. Use of confidence intervals in analysis of comparative bioavailability trials. Journal of Pharmaceutical Sciences 1972; 61(8): 1340–1341. - PubMed
-
- Westlake WJ. Symmetrical confidence intervals for bioequivalence trials. Biometrics 1976: 741–744. - PubMed
-
- Anderson S, Hauck WW. A new procedure for testing equivalence in comparative bioavailability and other clinical trials. Communications in Statistics-Theory and Methods 1983; 12(23): 2663–2692.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
