A Phase 1 study of GDC-0134, a dual leucine zipper kinase inhibitor, in ALS
- PMID: 35014217
- PMCID: PMC8791798
- DOI: 10.1002/acn3.51491
A Phase 1 study of GDC-0134, a dual leucine zipper kinase inhibitor, in ALS
Abstract
Objective: Dual leucine zipper kinase (DLK), which regulates the c-Jun N-terminal kinase pathway involved in axon degeneration and apoptosis following neuronal injury, is a potential therapeutic target in amyotrophic lateral sclerosis (ALS). This first-in-human study investigated safety, tolerability, and pharmacokinetics (PK) of oral GDC-0134, a small-molecule DLK inhibitor. Plasma neurofilament light chain (NFL) levels were explored in GDC-0134-treated ALS patients and DLK conditional knockout (cKO) mice.
Methods: The study included placebo-controlled, single and multiple ascending-dose (SAD; MAD) stages, and an open-label safety expansion (OLE) with adaptive dosing for up to 48 weeks.
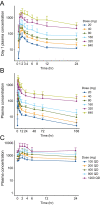
Results: Forty-nine patients were enrolled. GDC-0134 (up to 1200 mg daily) was well tolerated in the SAD and MAD stages, with no serious adverse events (SAEs). In the OLE, three study drug-related SAEs occurred: thrombocytopenia, dysesthesia (both Grade 3), and optic ischemic neuropathy (Grade 4); Grade ≤2 sensory neurological AEs led to dose reductions/discontinuations. GDC-0134 exposure was dose-proportional (median half-life = 84 h). Patients showed GDC-0134 exposure-dependent plasma NFL elevations; DLK cKO mice also exhibited plasma NFL compared to wild-type littermates.
Interpretation: This trial characterized GDC-0134 safety and PK, but no adequately tolerated dose was identified. NFL elevations in GDC-0134-treated patients and DLK cKO mice raised questions about interpretation of biomarkers affected by both disease and on-target drug effects. The safety profile of GDC-0134 was considered unacceptable and led to discontinuation of further drug development for ALS. Further work is necessary to understand relationships between neuroprotective and potentially therapeutic effects of DLK knockout/inhibition and NFL changes in patients with ALS.
© 2022 Genentech, Inc. Annals of Clinical and Translational Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
JSK: Nothing to disclose. JDR: Nothing to disclose. MEC: Consultant for Avexis, consultant for Orion, consultant for Lilly, consultant for Biohaven, consultant for MT Pharma, consultant for Revalasio, consultant for Pontifex, consultant for Denali, consultant for Biogen, consultant for Pharm NEXT, consultant for Treeway, consultant for Takeda, consultant for Aclipse, consultant for Sunovian, consultant for Anelixis, consultant for Cytokinetics, consultant for Disarm, consultant for ALS Pharma, consultant for RRD, consultant for Immunity Pharma, consultant for Helixsmith, consultant for Wave, consultant for Transposon, consultant for Quralis. AG: Other from Quralis, other from Mitsubishi Tanabe Pharma America, other from Sanofi Genzyme, other from AL‐S Pharma, other from Biogen, other from Novartis, other from Uniqure, other from Affinia, other from Apellis, other from Avenix, other from Alexion, other from Wave Life Sciences, other from Roche, other from Cytokinetics, other from Orion, other from Medtronic, other from Anaelixis, other from Viela, other from ArgenX, other from BMS, other from Sanofi Genzyme, other from Grifols, other from Ionis, other from Lily, other from Machinova, other from Novartis, other from Orphazyme, other from Pfizer, other from Ra Pharmaseuticals, other from Teva, other from UCB, during the conduct of the study. BO: Grants from Genentech, A Member of the Roche Group, during the conduct of the study; grants and personal fees from Biogen, grants from Eisai, grants and personal fees from Mitsubishi Tanabe, grants and personal fees from Medicinova, grants from AZ Therapeutics, personal fees from Biohaven, personal fees from Tsumura, outside the submitted work. ABH: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. CC: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. JG: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. BLB: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. WC: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. GAK: Employee of F. Hoffmann‐La Roche, Ltd. FLY: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. ASG: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. SC: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. LB: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. LH: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. JAC: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. MER: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. FB: Employee of Genentech, Inc. and shareholder in F. Hoffmann‐La Roche, Ltd. KRS: Nothing to disclose. LvdB: Nothing to disclose. JDB: Grants from Genentech, during the conduct of the study; personal fees from Biogen, personal fees from Clene Nanomedicine, grants from Alexion, grants from Biogen, grants from MT Pharma of America, grants from Anelixis Therapeutics, grants from Brainstorm Cell Therapeutics, grants from nQ Medical, grants from NINDS, grants from Muscular Dystrophy Association, grants from ALS One, grants from Amylyx Therapeutics, personal fees from MT Pharma Holdings of America, grants from ALS Association, grants from ALS Finding A Cure, outside the submitted work. JDG: Emory University was a clinical trial site during the conduct of the study.
Figures

References
-
- Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942‐955. - PubMed
-
- Swinnen B, Robberecht W. The phenotypic variability of amyotrophic lateral sclerosis. Nat Rev Neurol. 2014;10:661‐670. - PubMed
-
- Mathis S, Goizet C, Soulages A, et al. Genetics of amyotrophic lateral sclerosis: a review. J Neurol Sci. 2019;399:217‐226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

