Stable flow-induced expression of KLK10 inhibits endothelial inflammation and atherosclerosis
- PMID: 35014606
- PMCID: PMC8806187
- DOI: 10.7554/eLife.72579
Stable flow-induced expression of KLK10 inhibits endothelial inflammation and atherosclerosis
Abstract
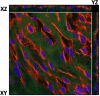
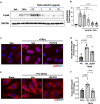
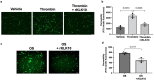
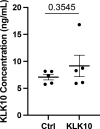
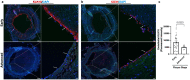
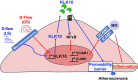
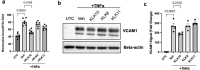
Atherosclerosis preferentially occurs in arterial regions exposed to disturbed blood flow (d-flow), while regions exposed to stable flow (s-flow) are protected. The proatherogenic and atheroprotective effects of d-flow and s-flow are mediated in part by the global changes in endothelial cell (EC) gene expression, which regulates endothelial dysfunction, inflammation, and atherosclerosis. Previously, we identified kallikrein-related peptidase 10 (Klk10, a secreted serine protease) as a flow-sensitive gene in mouse arterial ECs, but its role in endothelial biology and atherosclerosis was unknown. Here, we show that KLK10 is upregulated under s-flow conditions and downregulated under d-flow conditions using in vivo mouse models and in vitro studies with cultured ECs. Single-cell RNA sequencing (scRNAseq) and scATAC sequencing (scATACseq) study using the partial carotid ligation mouse model showed flow-regulated Klk10 expression at the epigenomic and transcription levels. Functionally, KLK10 protected against d-flow-induced permeability dysfunction and inflammation in human artery ECs, as determined by NFκB activation, expression of vascular cell adhesion molecule 1 and intracellular adhesion molecule 1, and monocyte adhesion. Furthermore, treatment of mice in vivo with rKLK10 decreased arterial endothelial inflammation in d-flow regions. Additionally, rKLK10 injection or ultrasound-mediated transfection of Klk10-expressing plasmids inhibited atherosclerosis in Apoe-/- mice. Moreover, KLK10 expression was significantly reduced in human coronary arteries with advanced atherosclerotic plaques compared to those with less severe plaques. KLK10 is a flow-sensitive endothelial protein that serves as an anti-inflammatory, barrier-protective, and anti-atherogenic factor.
Keywords: atherosclerosis; cell biology; endothelial cells; human; immunology; inflammation; kallikreins; mechanobiology; mouse; shear stress.
Conflict of interest statement
DW, MM, RL, AA, SK, DK, JZ, IT, NV, KB, LZ, ET, PB, JP, LM, KM, MH, ZD No competing interests declared, HL, YA is affiliated with Celltrion. The author has no financial interests to declare, ED has consulted for Abbott diagnostics and Imaware Disgnostics. The author has no other competing interest to declare, HJ is the founder of FloKines Pharma
Figures









References
-
- Alberts-Grill N, Rezvan A, Son DJ, Qiu H, Kim CW, Kemp ML, Weyand CM, Jo H. Dynamic immune cell accumulation during flow-induced atherogenesis in mouse carotid artery: an expanded flow cytometry method. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32:623–632. doi: 10.1161/ATVBAHA.111.242180. - DOI - PMC - PubMed
-
- Baeriswyl DC, Prionisti I, Peach T, Tsolkas G, Chooi KY, Vardakis J, Morel S, Diagbouga MR, Bijlenga P, Cuhlmann S, Evans P, Kwak BR, Ventikos Y, Krams R. Disturbed flow induces a sustained, stochastic NF-κB activation which may support intracranial aneurysm growth in vivo. Scientific Reports. 2019;9:1–14. doi: 10.1038/s41598-019-40959-y. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

