Inhibition of FOXO1‑mediated autophagy promotes paclitaxel‑induced apoptosis of MDA‑MB‑231 cells
- PMID: 35014689
- PMCID: PMC8767459
- DOI: 10.3892/mmr.2022.12588
Inhibition of FOXO1‑mediated autophagy promotes paclitaxel‑induced apoptosis of MDA‑MB‑231 cells
Abstract
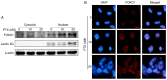
Triple‑negative breast cancer (TNBC) is the most aggressive subtype of breast cancer, and it often becomes resistant to paclitaxel (PTX) therapy. Autophagy plays an important cytoprotective role in PTX‑induced tumor cell death, and targeting autophagy has been promising for improving the efficacy of tumor chemotherapy in recent years. The aim of the present study was to clarify the mechanism of PTX inducing autophagy in TNBC cells to provide a potential clinical chemotherapy strategy of PTX for TNBC. The present study reported that PTX induced both apoptosis and autophagy in MDA‑MB‑231 cells and that inhibition of autophagy promoted apoptotic cell death. Furthermore, it was found that forkhead box transcription factor O1 (FOXO1) enhanced PTX‑induced autophagy through a transcriptional activation pattern in MDA‑MB‑231 cells, which was associated with the downstream target genes autophagy related 5, class III phosphoinositide 3‑kinase vacuolar protein sorting 34, autophagy related 4B cysteine peptidase, beclin 1 and microtubule associated protein 1 light chain 3β. Knocking down FOXO1 attenuated the survival of MDA‑MB‑231 cells in response to PTX treatment. These findings may be beneficial for improving the treatment efficacy of PTX and to develop autophagic targeted therapy for TNBC.
Keywords: apoptosis; autophagy; forkhead box transcription factor O1; paclitaxel; triple‑negative breast cancer.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Lock R, Kenific CM, Leidal AM, Salas E, Debnath J. Autophagy-dependent production of secreted factors facilitates oncogenic RAS-driven invasion. Cancer Discov. 2014;4:466–479. doi: 10.1158/2159-8290.CD-13-0841. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

