Templation and Concentration Drive Conversion Between a FeII12L12 Pseudoicosahedron, a FeII4L4 Tetrahedron, and a FeII2L3 Helicate
- PMID: 35014803
- PMCID: PMC9097479
- DOI: 10.1021/jacs.1c11536
Templation and Concentration Drive Conversion Between a FeII12L12 Pseudoicosahedron, a FeII4L4 Tetrahedron, and a FeII2L3 Helicate
Abstract
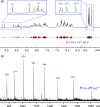
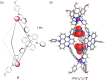
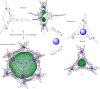
We report the construction of three structurally distinct self-assembled architectures: FeII12L12 pseudoicosahedron 1, FeII2L3 helicate 2, and FeII4L4 tetrahedron 3, formed from a single triazatriangulenium subcomponent A under different reaction conditions. Pseudoicosahedral capsule 1 is the largest formed through subcomponent self-assembly to date, with an outer-sphere diameter of 5.4 nm and a cavity volume of 15 nm3. The outcome of self-assembly depended upon concentration, where the formation of pseudoicosahedron 1 was favored at higher concentrations, while helicate 2 exclusively formed at lower concentrations. The conversion of pseudoicosahedron 1 or helicate 2 into tetrahedron 3 occurred following the addition of a CB11H12- or B12F122- template.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Burke B. P.; Grantham W.; Burke M. J.; Nichol G. S.; Roberts D.; Renard I.; Hargreaves R.; Cawthorne C.; Archibald S. J.; Lusby P. J. Visualizing kinetically robust CoIII4L6 assemblies in vivo: SPECT imaging of the encapsulated [99mTc]TcO4– anion. J. Am. Chem. Soc. 2018, 140, 16877–16881. 10.1021/jacs.8b09582. - DOI - PubMed
- Ozores H. L.; Amorin M.; Granja J. R. Self-assembling molecular capsules based on α,γ-cyclic peptides. J. Am. Chem. Soc. 2017, 139, 776–784. 10.1021/jacs.6b10456. - DOI - PubMed
- Akine S.; Miyashita M.; Nabeshima T. A closed metallomolecular cage that can open its aperture by disulfide exchange. Chem.—Eur. J. 2019, 25, 1432–1435. 10.1002/chem.201805359. - DOI - PubMed
- Mohankumar M.; Holler M.; Meichsner E.; Nierengarten J. F.; Niess F.; Sauvage J. P.; Delavaux-Nicot B.; Leoni E.; Monti F.; Malicka J. M.; Cocchi M.; Bandini E.; Armaroli N. Heteroleptic copper(I) pseudorotaxanes incorporating macrocyclic phenanthroline ligands of different sizes. J. Am. Chem. Soc. 2018, 140, 2336–2347. 10.1021/jacs.7b12671. - DOI - PubMed
- Han Y. F.; Jia W. G.; Yu W. B.; Jin G. X. Stepwise formation of organometallic macrocycles, prisms and boxes from Ir, Rh and Ru-based half-sandwich units. Chem. Soc. Rev. 2009, 38, 3419–3434. 10.1039/b901649j. - DOI - PubMed
- Jiao J.; Tan C.; Li Z.; Liu Y.; Han X.; Cui Y. Design and assembly of chiral coordination cages for asymmetric sequential reactions. J. Am. Chem. Soc. 2018, 140, 2251–2259. 10.1021/jacs.7b11679. - DOI - PubMed
- Hou Y. J.; Wu K.; Wei Z. W.; Li K.; Lu Y. L.; Zhu C. Y.; Wang J. S.; Pan M.; Jiang J. J.; Li G. Q.; Su C. Y. Design and enantioresolution of homochiral Fe(II)-Pd(II) coordination cages from stereolabile metalloligands: stereochemical stability and enantioselective separation. J. Am. Chem. Soc. 2018, 140, 18183–18191. 10.1021/jacs.8b11152. - DOI - PubMed
- Chen L. J.; Yang H. B.; Shionoya M. Chiral metallosupramolecular architectures. Chem. Soc. Rev. 2017, 46, 2555–2576. 10.1039/C7CS00173H. - DOI - PubMed
-
- Chan A. K.; Lam W. H.; Tanaka Y.; Wong K. M.; Yam V. W. Multiaddressable molecular rectangles with reversible host-guest interactions: modulation of pH-controlled guest release and capture. Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 690–695. 10.1073/pnas.1423709112. - DOI - PMC - PubMed
- Bloch W. M.; Abe Y.; Holstein J. J.; Wandtke C. M.; Dittrich B.; Clever G. H. Geometric complementarity in assembly and guest recognition of a bent heteroleptic cis-[Pd2LA2LB2] coordination cage. J. Am. Chem. Soc. 2016, 138, 13750–13755. 10.1021/jacs.6b08694. - DOI - PubMed
- Zhang Z.; Kim D. S.; Lin C. Y.; Zhang H.; Lammer A. D.; Lynch V. M.; Popov I.; Miljanic O. S.; Anslyn E. V.; Sessler J. L. Expanded porphyrin-anion supramolecular assemblies: environmentally responsive sensors for organic solvents and anions. J. Am. Chem. Soc. 2015, 137, 7769–7774. 10.1021/jacs.5b03131. - DOI - PubMed
- Custelcean R.; Bonnesen P. V.; Duncan N. C.; Zhang X.; Watson L. A.; Van Berkel G.; Parson W. B.; Hay B. P. Urea-functionalized M4L6 cage receptors: anion-templated self-assembly and selective guest exchange in aqueous solutions. J. Am. Chem. Soc. 2012, 134, 8525–8534. 10.1021/ja300677w. - DOI - PubMed
- Samanta S. K.; Moncelet D.; Briken V.; Isaacs L. Metal–organic polyhedron capped with cucurbit[8]uril delivers doxorubicin to cancer cells. J. Am. Chem. Soc. 2016, 138, 14488–14496. 10.1021/jacs.6b09504. - DOI - PMC - PubMed
- Bruns C. J.; Fujita D.; Hoshino M.; Sato S.; Stoddart J. F.; Fujita M. Emergent ion-gated binding of cationic host-guest complexes within cationic M12L24 molecular flasks. J. Am. Chem. Soc. 2014, 136, 12027–12034. 10.1021/ja505296e. - DOI - PubMed
-
- You L.; Berman J. S.; Anslyn E. V. Dynamic multi-component covalent assembly for the reversible binding of secondary alcohols and chirality sensing. Nat. Chem. 2011, 3, 943–948. 10.1038/nchem.1198. - DOI - PMC - PubMed
- Albrecht M.; Isaak E.; Baumert M.; Gossen V.; Raabe G.; Fröhlich R. “Induced fit” in chiral recognition: epimerization upon dimerization in the hierarchical self-assembly of helicate-type titanium(IV) complexes. Angew. Chem., Int. Ed. 2011, 50, 2850–2853. 10.1002/anie.201006448. - DOI - PubMed
-
- Zhang D.; Ronson T. K.; Zou Y.-Q.; Nitschke J. R. Metal–organic cages for molecular separations. Nat. Rev. Chem. 2021, 5, 168–182. 10.1038/s41570-020-00246-1. - DOI - PubMed
- Fuertes-Espinosa C.; Gomez-Torres A.; Morales-Martinez R.; Rodriguez-Fortea A.; Garcia-Simon C.; Gandara F.; Imaz I.; Juanhuix J.; Maspoch D.; Poblet J. M.; Echegoyen L.; Ribas X. Purification of uranium-based endohedral metallofullerenes (EMFs) by selective supramolecular encapsulation and release. Angew. Chem., Int. Ed. 2018, 57, 11294–11299. 10.1002/anie.201806140. - DOI - PubMed
- Catti L.; Zhang Q.; Tiefenbacher K. Advantages of catalysis in self-assembled molecular capsules. Chem.—Eur. J. 2016, 22, 9060–9066. 10.1002/chem.201600726. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

