Effect of Anticoagulant Therapy for 6 Weeks vs 3 Months on Recurrence and Bleeding Events in Patients Younger Than 21 Years of Age With Provoked Venous Thromboembolism: The Kids-DOTT Randomized Clinical Trial
- PMID: 35015038
- PMCID: PMC8753509
- DOI: 10.1001/jama.2021.23182
Effect of Anticoagulant Therapy for 6 Weeks vs 3 Months on Recurrence and Bleeding Events in Patients Younger Than 21 Years of Age With Provoked Venous Thromboembolism: The Kids-DOTT Randomized Clinical Trial
Erratum in
-
Incorrect Placement of Boxes in Figure 1 and Addition of a Primary Investigator.JAMA. 2022 Mar 22;327(12):1188. doi: 10.1001/jama.2022.3496. JAMA. 2022. PMID: 35315909 Free PMC article. No abstract available.
Abstract
Importance: Among patients younger than 21 years of age, the optimal duration of anticoagulant therapy for venous thromboembolism is unknown.
Objective: To test the hypothesis that a 6-week duration of anticoagulant therapy for provoked venous thromboembolism is noninferior to a conventional 3-month therapy duration in patients younger than 21 years of age.
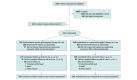
Design, setting, and participants: Randomized clinical trial involving 417 patients younger than 21 years of age with acute, provoked venous thromboembolism enrolled at 42 centers in 5 countries from 2008-2021. The main exclusions were severe anticoagulant deficiencies or prior venous thromboembolism. Patients without persistent antiphospholipid antibodies and whose thrombi were resolved or not completely occlusive upon repeat imaging at 6 weeks after diagnosis underwent randomization. The final visit for the primary end points occurred in January 2021.
Interventions: Total duration for anticoagulant therapy of 6 weeks (n = 207) vs 3 months (n = 210) for provoked venous thromboembolism.
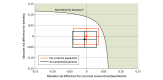
Main outcomes and measures: The primary efficacy and safety end points were centrally adjudicated symptomatic recurrent venous thromboembolism and clinically relevant bleeding events within 1 year blinded to treatment group. The primary analysis was noninferiority in the per-protocol population. The noninferiority boundary incorporated a bivariate trade-off that included an absolute increase of 0% in symptomatic recurrent venous thromboembolism with an absolute risk reduction of 4% in clinically relevant bleeding events (1 of 3 points on the bivariate noninferiority boundary curve).
Results: Among 417 randomized patients, 297 (median age, 8.3 [range, 0.04-20.9] years; 49% female) met criteria for the primary per-protocol population analysis. The Kaplan-Meier estimate for the 1-year cumulative incidence of the primary efficacy outcome was 0.66% (95% CI, 0%-1.95%) in the 6-week anticoagulant therapy group and 0.70% (95% CI, 0%-2.07%) in the 3-month anticoagulant therapy group, and for the primary safety outcome, the incidence was 0.65% (95% CI, 0%-1.91%) and 0.70% (95% CI, 0%-2.06%). Based on absolute risk differences in recurrent venous thromboembolism and clinically relevant bleeding events between groups, noninferiority was demonstrated. Adverse events occurred in 26% of patients in the 6-week anticoagulant therapy group and in 32% of patients in the 3-month anticoagulant therapy group; the most common adverse event was fever (1.9% and 3.4%, respectively).
Conclusions and relevance: Among patients younger than 21 years of age with provoked venous thromboembolism, anticoagulant therapy for 6 weeks compared with 3 months met noninferiority criteria based on the trade-off between recurrent venous thromboembolism risk and bleeding risk.
Trial registration: ClinicalTrials.gov Identifier: NCT00687882.
Conflict of interest statement
Figures
Comment in
-
Duration of Anticoagulant Treatment for Acute Provoked Venous Thromboembolism in Pediatric Patients.JAMA. 2022 Jan 11;327(2):124-125. doi: 10.1001/jama.2021.21890. JAMA. 2022. PMID: 35015051 No abstract available.
-
Anticoagulant Therapy for 6 Weeks vs 3 Months and Recurrence and Bleeding in Patients Younger Than 21 Years With Provoked Venous Thromboembolism.JAMA. 2022 May 3;327(17):1709. doi: 10.1001/jama.2022.3726. JAMA. 2022. PMID: 35503350 No abstract available.
References
-
- Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. Lancet. 1960;1(7138):1309-1312. - PubMed
-
- Kahn SR, Shrier I, Julian JA, et al. Determinants and time course of the postthrombotic syndrome after acute deep venous thrombosis. Ann Intern Med. 2008;149(10):698-707. - PubMed
-
- Prandoni P, Lensing AW, Cogo A, et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med. 1996;125(1):1-7. - PubMed
-
- Chi G, Goldhaber SZ, Kittelson JM, et al. Effect of extended-duration thromboprophylaxis on venous thromboembolism and major bleeding among acutely ill hospitalized medical patients. J Thromb Haemost. 2017;15(10):1913-1922. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

