Malondialdehyde-Modified Photoreceptor Outer Segments Promote Choroidal Neovascularization in Mice
- PMID: 35015060
- PMCID: PMC8762676
- DOI: 10.1167/tvst.11.1.12
Malondialdehyde-Modified Photoreceptor Outer Segments Promote Choroidal Neovascularization in Mice
Abstract
Purpose: This study aimed to establish a novel choroidal neovascularization (CNV) mouse model through subretinally injecting malondialdehyde (MDA)-modified photoreceptor outer segments (POS), which was more consistent with the pathogenesis of wet age-related macular degeneration (AMD).
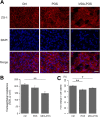
Methods: MDA-modified POS were subretinally injected in C57BL/6J mice. Four weeks later, to assess the volume of CNV and the morphology of retinal pigment epithelium (RPE), isolectin B4 and zonula occludens-1 antibody were used for immunostaining. Fundus fluorescent angiography and optical coherence tomography imaging were used to describe the morphologic features of CNV. Transepithelial resistance was measured on polarized ARPE-19 cells. Vascular endothelial growth factor levels in the cell culture medium were detected by enzyme-linked immunosorbent assay. The protein and messenger RNA expression levels of autophagy markers were measured using Western blot and quantitative polymerase chain reaction.
Results: CNV and RPE atrophy were successfully induced in the mouse model. MDA-modified POS also significantly increased the expression of vascular endothelial growth factor and disrupted cell junctions in RPE cells. In addition, MDA-modified POS induced autophagy-lysosomal impairment in RPE cells.
Conclusions: Subretinal injection of MDA-modified POS may generate a feasible CNV model that simulates the AMD pathological process.
Translational relevance: This study expands the understanding of the role of MDA in AMD pathogenesis, which provides a potential therapeutic target of AMD.
Conflict of interest statement
Disclosure:
Figures




References
-
- Mitchell P, Liew G, Gopinath B, et al. .. Age-related macular degeneration. Lancet. 2018; 392(10153): 1147–1159. - PubMed
-
- Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic Biol Med. 2009; 47(5): 469–484. - PubMed
-
- Uchida K. Aldehyde adducts generated during lipid peroxidation modification of proteins. Free Radic Res. 2015; 49(7): 896–904. - PubMed
-
- Del Rio D, Stewart AJ, Pellegrini N.. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005; 15(4): 316–328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

