Outcomes of SARS-CoV-2-Positive Youths Tested in Emergency Departments: The Global PERN-COVID-19 Study
- PMID: 35015063
- PMCID: PMC8753506
- DOI: 10.1001/jamanetworkopen.2021.42322
Outcomes of SARS-CoV-2-Positive Youths Tested in Emergency Departments: The Global PERN-COVID-19 Study
Abstract
Importance: Severe outcomes among youths with SARS-CoV-2 infections are poorly characterized.
Objective: To estimate the proportion of children with severe outcomes within 14 days of testing positive for SARS-CoV-2 in an emergency department (ED).
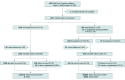
Design, setting, and participants: This prospective cohort study with 14-day follow-up enrolled participants between March 2020 and June 2021. Participants were youths aged younger than 18 years who were tested for SARS-CoV-2 infection at one of 41 EDs across 10 countries including Argentina, Australia, Canada, Costa Rica, Italy, New Zealand, Paraguay, Singapore, Spain, and the United States. Statistical analysis was performed from September to October 2021.
Exposures: Acute SARS-CoV-2 infection was determined by nucleic acid (eg, polymerase chain reaction) testing.
Main outcomes and measures: Severe outcomes, a composite measure defined as intensive interventions during hospitalization (eg, inotropic support, positive pressure ventilation), diagnoses indicating severe organ impairment, or death.
Results: Among 3222 enrolled youths who tested positive for SARS-CoV-2 infection, 3221 (>99.9%) had index visit outcome data available, 2007 (62.3%) were from the United States, 1694 (52.6%) were male, and 484 (15.0%) had a self-reported chronic illness; the median (IQR) age was 3 (0-10) years. After 14 days of follow-up, 735 children (22.8% [95% CI, 21.4%-24.3%]) were hospitalized, 107 (3.3% [95% CI, 2.7%-4.0%]) had severe outcomes, and 4 children (0.12% [95% CI, 0.03%-0.32%]) died. Characteristics associated with severe outcomes included being aged 5 to 18 years (age 5 to <10 years vs <1 year: odds ratio [OR], 1.60 [95% CI, 1.09-2.34]; age 10 to <18 years vs <1 year: OR, 2.39 [95% CI 1.38-4.14]), having a self-reported chronic illness (OR, 2.34 [95% CI, 1.59-3.44]), prior episode of pneumonia (OR, 3.15 [95% CI, 1.83-5.42]), symptoms starting 4 to 7 days prior to seeking ED care (vs starting 0-3 days before seeking care: OR, 2.22 [95% CI, 1.29-3.82]), and country (eg, Canada vs US: OR, 0.11 [95% CI, 0.05-0.23]; Costa Rica vs US: OR, 1.76 [95% CI, 1.05-2.96]; Spain vs US: OR, 0.51 [95% CI, 0.27-0.98]). Among a subgroup of 2510 participants discharged home from the ED after initial testing and who had complete follow-up, 50 (2.0%; 95% CI, 1.5%-2.6%) were eventually hospitalized and 12 (0.5%; 95% CI, 0.3%-0.8%) had severe outcomes. Compared with hospitalized SARS-CoV-2-negative youths, the risk of severe outcomes was higher among hospitalized SARS-CoV-2-positive youths (risk difference, 3.9%; 95% CI, 1.1%-6.9%).
Conclusions and relevance: In this study, approximately 3% of SARS-CoV-2-positive youths tested in EDs experienced severe outcomes within 2 weeks of their ED visit. Among children discharged home from the ED, the risk was much lower. Risk factors such as age, underlying chronic illness, and symptom duration may be useful to consider when making clinical care decisions.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

