Review of Over 15 Years Postmarketing Safety Surveillance Spontaneous Data for the Human Rotavirus Vaccine (Rotarix) on Intussusception
- PMID: 35015268
- PMCID: PMC8894299
- DOI: 10.1007/s40264-021-01141-4
Review of Over 15 Years Postmarketing Safety Surveillance Spontaneous Data for the Human Rotavirus Vaccine (Rotarix) on Intussusception
Erratum in
-
Correction to: Review of Over 15 Years Postmarketing Safety Surveillance Spontaneous Data for the Human Rotavirus Vaccine (Rotarix) on Intussusception.Drug Saf. 2022 Apr;45(4):401. doi: 10.1007/s40264-022-01167-2. Drug Saf. 2022. PMID: 35325445 Free PMC article. No abstract available.
Abstract
Introduction: Rotavirus (RV) is the most common cause of acute gastroenteritis in children <5 years of age worldwide, and vaccination reduces the disease burden. Evidence from postmarketing surveillance studies suggested an increased risk of intussusception (IS) in infants post-RV vaccination. An overall positive benefit-risk balance for the human RV vaccine (HRV) Rotarix (GlaxoSmithKline [GSK], Belgium) has been established and recent findings indicate an indirect effect of reduced IS over the long term.
Objective: The aim of this study was to discuss spontaneous data from the GSK worldwide safety database on IS post-Rotarix administration.
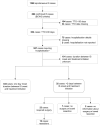
Methods: The database was reviewed for all spontaneous IS cases from 2004 to 2020. Additionally, an observed versus expected (O/E) analysis was done for adverse events attributed to IS. Data were reviewed as overall worldwide and stratified by region (Europe/USA/Japan) and dose.
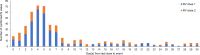
Results: A male predominance of IS patients was observed, consistent with earlier reports. The most frequently reported events in confirmed IS cases (Brighton Collaboration Working Group [BCWG] level 1) with time to onset ≤ 30 days post-vaccination were vomiting (55.8%), haematochezia (47.2%), and crying (21.1%). The observations from the IS spontaneous cases review and results of the O/E analysis are consistent with the known IS safety profile of RV vaccines: a transient increased incidence of IS post-vaccination (primarily in Europe/Japan/worldwide), mostly within 7 days postdose 1.
Conclusion: Since the outcomes of early IS management are favourable over delayed management, healthcare professionals should inform parents about the importance of seeking immediate medical advice in case of unusual behaviour of the vaccinated infant. GSK continues to monitor the IS risk post-Rotarix administration through routine pharmacovigilance activities.
Plain language summary
Rotavirus (RV) is the most common cause of acute gastroenteritis and a major cause of death in young children worldwide. Vaccination has been instrumental in reducing the impact of RV disease. Real-world evidence suggests an increased risk of intussusception (an infrequent type of bowel obstruction) in infants following RV vaccination. We reviewed IS cases reported spontaneously worldwide in children following a two-dose vaccination with the human RV vaccine (Rotarix, GlaxoSmithKline [GSK]) since its launch in 2004. We observed that (1) IS occurred more frequently 7 days after the first dose and, to a lesser extent, after the second dose; (2) boys were more frequently affected than girls (56.3%); (3) of 862 confirmed reported cases, 557 required hospitalisation; and (4) surgical intervention was required for 294 of 557 hospitalised cases. We used statistical analysis to assess whether the number of cases observed would be higher or lower than the natural occurrence of IS (irrespective of vaccination). These results were in line with the known RV vaccine safety profile. It is important to constantly monitor the real-world safety profile of RV vaccines in the postmarketing setting. Since the outcomes of early management of IS are favourable compared with delayed management, healthcare professionals should inform parents to seek immediate medical advice if they observe unusual behaviour in their vaccinated child. In conclusion, our analyses on data of a large patient pool for this rare event reinforce the favourable safety profile of human RV vaccine and the benefits of vaccination in young children.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
Tina Singh, Frédérique Delannois, François Haguinet, and Lifeter Yenwo Molo are employees of the GSK group of companies. Frédérique Delannois, François Haguinet, and Tina Singh hold shares in the GSK group of companies as part of their employee remuneration. The authors declare no other financial or non-financial conflicts of interest.
Figures
Similar articles
-
Post-marketing monitoring of intussusception after rotavirus vaccination in Japan.Pharmacoepidemiol Drug Saf. 2015 Jul;24(7):765-70. doi: 10.1002/pds.3800. Epub 2015 May 27. Pharmacoepidemiol Drug Saf. 2015. PMID: 26013569 Free PMC article.
-
Post-marketing surveillance of intussusception after Rotarix administration in Afghanistan, 2018-2022.Vaccine. 2024 Mar 19;42(8):2059-2064. doi: 10.1016/j.vaccine.2024.02.057. Epub 2024 Feb 26. Vaccine. 2024. PMID: 38413278 Free PMC article.
-
Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico.Pediatr Infect Dis J. 2012 Jul;31(7):736-44. doi: 10.1097/INF.0b013e318253add3. Pediatr Infect Dis J. 2012. PMID: 22695189
-
Dynamics of G2P[4] strain evolution and rotavirus vaccination: A review of evidence for Rotarix.Vaccine. 2020 Jul 31;38(35):5591-5600. doi: 10.1016/j.vaccine.2020.06.059. Epub 2020 Jul 7. Vaccine. 2020. PMID: 32651115 Review.
-
Intussusception following rotavirus vaccination: an updated review of the available evidence.Expert Rev Vaccines. 2014 Nov;13(11):1339-48. doi: 10.1586/14760584.2014.942223. Epub 2014 Jul 26. Expert Rev Vaccines. 2014. PMID: 25066368 Review.
Cited by
-
Safety and Immunogenicity of a New Rotavirus-Inactivated Vaccine in the Chinese Adolescent Population: A Randomized, Double-Blind, Placebo-Controlled Phase I Clinical Trial.Vaccines (Basel). 2025 Mar 30;13(4):369. doi: 10.3390/vaccines13040369. Vaccines (Basel). 2025. PMID: 40333214 Free PMC article.
-
Attitudes and Beliefs towards Rotavirus Vaccination in a Sample of Italian Women: A Cross-Sectional Study.Vaccines (Basel). 2023 May 30;11(6):1041. doi: 10.3390/vaccines11061041. Vaccines (Basel). 2023. PMID: 37376430 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

