Immunoglobulin for multifocal motor neuropathy
- PMID: 35015296
- PMCID: PMC8751207
- DOI: 10.1002/14651858.CD004429.pub3
Immunoglobulin for multifocal motor neuropathy
Abstract
Background: Multifocal motor neuropathy (MMN) is a rare, probably immune-mediated disorder characterised by slowly progressive, asymmetric, distal weakness of one or more limbs with no objective loss of sensation. It may cause prolonged periods of disability. Treatment options for MMN are few. People with MMN do not usually respond to steroids or plasma exchange. Uncontrolled studies have suggested a beneficial effect of intravenous immunoglobulin (IVIg). This is an update of a Cochrane Review first published in 2005, with an amendment in 2007. We updated the review to incorporate new evidence.
Objectives: To assess the efficacy and safety of intravenous and subcutaneous immunoglobulin in people with MMN.
Search methods: We searched the following databases on 20 April 2021: the Cochrane Neuromuscular Specialised Register, CENTRAL, MEDLINE, Embase, ClinicalTrials.gov, and WHO ICTRP for randomised controlled trials (RCTs) and quasi-RCTs, and checked the reference lists of included studies.
Selection criteria: We considered RCTs and quasi-RCTs examining the effects of any dose of IVIg and subcutaneous immunoglobulin (SCIg) in people with definite or probable MMN for inclusion in the review. Eligible studies had to have measured at least one of the following outcomes: disability, muscle strength, or electrophysiological conduction block. We used studies that reported the frequency of adverse effects to assess safety.
Data collection and analysis: Two review authors independently reviewed the literature searches to identify potentially relevant trials, assessed risk of bias of included studies, and extracted data. We followed standard Cochrane methodology.
Main results: Six cross-over RCTs including a total of 90 participants were suitable for inclusion in the review. Five RCTs compared IVIg to placebo, and one compared IVIg to SCIg. Four of the trials comparing IVIg versus placebo involved IVIg-naive participants (induction treatment). In the other two trials, participants were known IVIg responders receiving maintencance IVIg at baseline and were then randomised to maintenance treatment with IVIg or placebo in one trial, and IVIg or SCIg in the other. Risk of bias was variable in the included studies, with three studies at high risk of bias in at least one risk of bias domain. IVIg versus placebo (induction treatment): three RCTs including IVIg-naive participants reported a disability measure. Disability improved in seven out of 18 (39%) participants after IVIg treatment and in two out of 18 (11%) participants after placebo (risk ratio (RR) 3.00, 95% confidence interval (CI) 0.89 to 10.12; 3 RCTs, 18 participants; low-certainty evidence). The proportion of participants with an improvement in disability at 12 months was not reported. Strength improved in 21 out of 27 (78%) IVIg-naive participants treated with IVIg and one out of 27 (4%) participants who received placebo (RR 11.00, 95% CI 2.86 to 42.25; 3 RCTs, 27 participants; low-certainty evidence). IVIg treatment may increase the proportion of people with resolution of at least one conduction block; however, the results were also consistent with no effect (RR 7.00, 95% CI 0.95 to 51.70; 4 RCTs, 28 participants; low-certainty evidence). IVIg versus placebo (maintenance treatment): a trial that included participants on maintenance IVIg treatment reported an increase in disability in 17 out of 42 (40%) people switching to placebo and seven out of 42 (17%) remaining on IVIg (RR 2.43, 95% CI 1.13 to 5.24; 1 RCT, 42 participants; moderate-certainty evidence) and a decrease in grip strength in 20 out of 42 (48%) participants after a switch to placebo treatment compared to four out of 42 (10%) remaining on IVIg (RR 0.20, 95% CI 0.07 to 0.54; 1 RCT, 42 participants; moderate-certainty evidence). Adverse events, IVIg versus placebo (induction or maintenance): four trials comparing IVIg and placebo reported adverse events, of which data from two studies could be meta-analysed. Transient side effects were reported in 71% of IVIg-treated participants versus 4.8% of placebo-treated participants in these studies. The pooled RR for the development of side effects was 10.33 (95% CI 2.15 to 49.77; 2 RCTs, 21 participants; very low-certainty evidence). There was only one serious side effect (pulmonary embolism) during IVIg treatment. IVIg versus SCIg (maintenance treatment): the trial that compared continuation of IVIg maintenance versus SCIg maintenance did not measure disability. The evidence was very uncertain for muscle strength (standardised mean difference 0.08, 95% CI -0.84 to 1.00; 1 RCT, 9 participants; very low-certainty evidence). The evidence was very uncertain for the number of people with side effects attributable to treatment (RR 0.50, 95% CI 0.18 to 1.40; 1 RCT, 9 participants; very low-certainty evidence).
Authors' conclusions: Low-certainty evidence from three small RCTs shows that IVIg may improve muscle strength in people with MMN, and low-certainty evidence indicates that it may improve disability; the estimate of the magnitude of improvement of disability has wide CIs and needs further studies to secure its significance. Based on moderate-certainty evidence, it is probable that most IVIg responders deteriorate in disability and muscle strength after IVIg withdrawal. SCIg might be an alternative treatment to IVIg, but the evidence is very uncertain. More research is needed to identify people in whom IVIg withdrawal is possible and to confirm efficacy of SCIg as an alternative maintenance treatment.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Stephen Keddie: is employed by Barts and the Royal London NHS Trust and University College London Hospitals NHS trust bank for clinical neurology work. Dr Keddie's research is funded by a clinical training research fellowship awarded by the Association of British Neurologists and Guarantors of Brain. Dr Keddie is a neurology specialist registrar and manages patients with multifocal motor neuropathy.
Filip Eftimov: Dr Eftimov received support for printing of his thesis from Sanquin Bloedvoorziening (Dutch blood bank and IVIg manufacturer) and CSL‐Behring in 2014. He reports lecture fees from CSL‐Behring, Kedrion, and Grifols. Outside of the submitted work, as principal investigator of INCbase, he also reports investigator‐initiated grants from Kedrion, Terumo BCT, CSL‐Behring, and Takeda Pharmaceutical Company, and grants from ZonMw (Dutch governmental agency) and Prinses Beatrix Spierfonds (a Dutch charity). In addition, his institution has received fees from UCB Pharma, CSL‐Behring, and Takeda for advisory board membership. All grants and fees were paid to his institution. He is a member of the Cochrane Neuromuscular Editorial Board.
Leonard H van den Berg: has co‐ordinated one randomised controlled trial of IVIg in multifocal motor neuropathy, which is included in this review. He received remuneration for sitting on scientific advisory boards of Baxter and Biogen Idec. His institution has received financial support from Baxter for organisation of an multifocal motor neuropathy masterclass.
Ruth Brassington: none known. She is Managing Editor of Cochrane Neuromuscular. She relinquished editorial responsibility for the review after joining the author team.
Rob J de Haan: none known.
Ivo N van Schaik: chaired a steering committee for a CSL‐Behring study investigating the safety and efficacy of SCIg in chronic inflammatory demyelinating polyradiculoneuropathy and received departmental honoraria for serving on scientific advisory boards for CSL‐Behring and Kedrion. He received departmental research support from the Netherlands Organisation for Scientific Research and the Dutch Prinses Beatrix Fonds. All lecturing and consulting fees for INvS were donated to the Stichting Klinische Neurologie, a local foundation that supports research in the field of neurological disorders. INvS served on the editorial board of Cochrane Neuromuscular, was a member of the organising committee of the Inflammatory Neuropathy Consortium (INC), a standing committee of the Peripheral Nerve Society, and was a member of the Scientific Board of the Kreuth III meeting on the optimal use of plasma‐derived medicinal products, especially coagulation factors and normal immunoglobulins, organised under the auspices of the European Directorate for the Quality of Medicines & HealthCare (EDQM).
This review does not comply with the relevant Cochrane Conflicts of Interest policy, revised in 2014, which requires the majority of review authors to have no relevant conflicts of interest. The review will be updated a year after publication, when the review will comply with the policy.
Figures
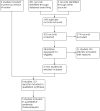














Update of
-
Intravenous immunoglobulin for multifocal motor neuropathy.Cochrane Database Syst Rev. 2005 Apr 18;(2):CD004429. doi: 10.1002/14651858.CD004429.pub2. Cochrane Database Syst Rev. 2005. Update in: Cochrane Database Syst Rev. 2022 Jan 11;1:CD004429. doi: 10.1002/14651858.CD004429.pub3. PMID: 15846714 Updated.
References
References to studies included in this review
Azulay 1994 {published data only}
-
- Azulay JP, Blin O, Pouget J, Boucraut J, Billé-Turc F, Carles G, et al. Intravenous immunoglobulin treatment in patients with motor neuron syndromes associated with anti-GM1 antibodies: a double-blind, placebo-controlled study. Neurology 1994;44(3 Pt 1):429-32. [PMID: ] - PubMed
Federico 2000 {published data only}
-
- Federico P, Zochodne DW, Feasby TE. Intravenous immunoglobulin treatment in multifocal motor neuropathy with conduction block: a double-blind, placebo-controlled, cross-over study. Neurology 1999;52(Suppl 2):A127.
-
- Federico P, Zochodne DW, Hahn AF, Brown WF, Feasby TE. Multifocal motor neuropathy improved by IVIg: randomized, double-blind, placebo-controlled study. Neurology 2000;55(9):1256-62. [PMID: ] - PubMed
Hahn 2013 {published data only}
-
- EUCTR2009-013841-27-DK. A randomized, double-blind, placebo controlled, cross-over study of the effectiveness of immune globulin intravenous (human), 10% (IGIV, 10%) for the treatment of multifocal motor neuropathy - IGIV, 10% MMN Trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009-013841-27-DK (first received 27 October 2009).
-
- Hahn AF, Beydoun SR, Lawson V, Oh M, Empson VG, Gelmont D, et al. A phase III, randomized, placebo-controlled study of the efficacy and safety of 10% liquid intravenous immunoglobulin (IVIG) for the treatment of multifocal motor neuropathy (MMN). Journal of the Peripheral Nervous System 2012;17(2):243-4.
-
- Hahn AF, Beydoun SR, Lawson V, for The IVIG in MMN Study Team, Oh M, Empson VG, Leibl H, et al. A controlled trial of intravenous immunoglobulin in multifocal motor neuropathy. Journal of the Peripheral Nervous System 2013;18(4):321-30. [EMBASE: 370497088] - PubMed
-
- Koski CL, Beydoun SR, Lawson V, Oh M, Gelmont D, Empson VG. A phase III, randomized, placebo-controlled study of the efficacy and safety of 10% liquid intravenous immunoglobulin (IGIV) for the treatment of multifocal motor neuropathy (MMN). PM & R: Journal of Injury, Function, and Rehabilitation 2013;5(9S):S152.
-
- NCT00666263. Study of the effectiveness of intravenous immune globulin (10%) for the treatment of multifocal motor neuropathy. clinicaltrials.gov/ct2/show/study/NCT00666263 (first received 24 April 2008).
Harbo 2009 {published data only}
-
- Harbo T, Andersen H, Hess A, Hansen K, Sindrup SH, Jakobsen J. Subcutaneous versus intravenous immunoglobulin in multifocal motor neuropathy: a randomized, single-blinded cross-over trial. European Journal of Neurology 2009;16(5):631-8. [PMID: ] - PubMed
-
- Harbo T, Andersen H, Sindrup S, Hansen K, Jakobsen J. Subcutaneous versus intravenous immunoglobulin treatment for MMN patients: a randomized, single-blinded, cross-over study. European Journal of Neurology 2008;15(Suppl 3):15-6. - PubMed
-
- NCT00268788. Subcutaneous immunoglobulin treatment for multifocal motor neuropathy. www.clinicaltrials.gov/ct2/show/NCT00268788 (first received 22 December 2005).
Léger 2001 {published data only}
-
- Léger JM, Chassande B, Musset L, Meininger V, Bouche P, Baumann N. Intravenous immunoglobulin therapy in multifocal motor neuropathy: a double-blind, placebo-controlled study. Brain 2001;124(1):145-53. [PMID: ] - PubMed
Van den Berg 1995 {published and unpublished data}
References to studies excluded from this review
Al‐Zuhairy 2019 {published data only}
-
- Al-Zuhairy A, Jakobsen J, Andersen H, Sindrup SH, Markvardsen LH. Facilitated subcutaneous immunoglobulin in multifocal motor neuropathy - a randomized, single-blinded, noninferiority cross-over trial. European Journal of Neurology 2019;26(S1):71. - PubMed
-
- Al-Zuhairy A, Jakobsen J, Andersen H, Sindrup SH, Markvardsen LK. Randomized trial of facilitated subcutaneous immunoglobulin in multifocal motor neuropathy. European Journal of Neurology 2019;26(10):1289-e82. - PubMed
-
- EUCTR2015-003453-18-DK. Study of the effect and safety of a new immunoglobulin preparation (HyQvia) for subcutaneous usage in patients with multifocal motor neuropathy. www.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2015-003453-18-DK (first received 23 September 2015).
-
- NCT02556437. Efficacy and safety of HyQvia (Immunoglobulin 10% with recombinant hyaluronidase) in multifocal motor neuropathy (MMN). clinicaltrials.gov/ct2/show/NCT02556437 (first received 22 September 2015).
Auer 1994 {published data only}
-
- Auer RN. Multifocal motor neuropathy. Annals of Neurology 1994;35(2):246-7. - PubMed
Baumann 2009 {published data only}
-
- Baumann A, Hess CW, Sturzenegger M. IVIg dose increase in multifocal motor neuropathy: a prospective six month follow-up. Journal of Neurology 2009;256(4):608-14. - PubMed
Burrell 2011 {published data only}
Cats 2008 {published data only}
-
- Cats EA, Pol WL, Piepers S, Notermans NC, den Berg-Vos RM, den Berg LH. New liquid intravenous immunoglobulin (10% IVIg) for treatment of multifocal motor neuropathy: a prospective study of efficacy, safety and tolerability. Journal of Neurology 2008;255(10):1598-9. - PubMed
Cats 2010 {published data only}
-
- Cats EA, Pol WL, Piepers S, Franssen H, Jacobs BC, den Berg-Vos RM, et al. Correlates of outcome and response to IVIg in 88 patients with multifocal motor neuropathy. Neurology 2010;75(9):818-25. - PubMed
Charles 1992 {published data only}
-
- Charles N, Benoit P, Vial C, Bierme T, Moreau T, Bady B. Intravenous immunoglobulin treatment in multifocal motor neuropathy. Lancet 1992;340(8812):182. - PubMed
Eftimov 2009 {published data only}
-
- Eftimov F, Vermeulen M, De Haan RJ, Van den Berg LH, Van Schaik IN. Subcutaneous immunoglobulin therapy for multifocal motor neuropathy. Journal of the Peripheral Nervous System 2009;14(2):93-100. - PubMed
Herraets 2019 {published data only}
Jafari 2000 {published data only}
-
- Jafari H, Carlander B, Camu W. Monofocal motor neuropathy responsive to intravenous immunoglobulins. Muscle & Nerve 2000;23(10):1610-11. - PubMed
Kelly 1992 {published data only}
-
- Kelly JJ Jr. Multifocal motor neuropathy. Neurology 1992;42(11):2230. - PubMed
Kermode 1992 {published data only}
-
- Kermode AG, Laing BA, Carroll WM, Mastaglia FL. Intravenous immunoglobulin for multifocal motor neuropathy. Lancet 1992;340(8824):920-1. - PubMed
Köller 2006 {published data only}
-
- Köller H, Schroeter M, Feischen H, Hartung HP, Kieseier BC. Subcutaneous self-infusions of immunoglobulins as a potential therapeutic regimen in immune-mediated neuropathies. Journal of Neurology 2006;253(11):1505-6. - PubMed
Komiyama 1998 {published data only}
-
- Komiyama A, Toda H, Hasegawa O, Kuroiwa Y. Multifocal motor neuropathy. Neurology 1998;50(1):314-5. - PubMed
Kubori 1999 {published data only}
-
- Kubori T, Mezaki T, Kaji R, Kimura J, Hamaguchi K, Hirayama K, et al. The clinical usefulness of high-dose intravenous immunoglobulin therapy for chronic inflammatory demyelinating polyneuropathy and multifocal motor neuropathy. No-to Shinkei [Brain & Nerve] 1999;51(2):127-35. - PubMed
Kuwabara 2018 {published data only}
-
- NCT01827072. Phase III clinical trial of NPB-01 maintenance therapy in patients with multifocal motor neuropathy. clinicaltrials.gov/ct2/show/NCT01827072 (first received 9 April 2018).
Léger 2008 {published data only}
-
- Léger JM, Viala K, Cancalon F, Maisonobe T, Gruwez B, Waegemans T, et al. Intravenous immunoglobulin as short- and long-term therapy of multifocal motor neuropathy: a retrospective study of response to IVIg and of its predictive criteria in 40 patients. Journal of Neurology, Neurosurgery and Psychiatry 2008;79(1):93-6. - PubMed
Léger 2019 {published data only}
-
- EUCTR2012-01995-12-ES. A European, randomised, double-blind, active comparator-controlled, cross-over, efficacy and safety study of a new 10% ready-to-use liquid human intravenous immunoglobulin (I10E) versus Kiovig® in patients with multifocal motor neuropathy. www.clinicaltrialsregister.eu/ctr-search/trial/2012-001995-12/GB (first received 3 June 2013).
-
- Léger JM, Alfa Cissé O, Cocito D, Grouin JM, Katifi H, Nobile-Orazio E, et al. IqYmune® is an effective maintenance treatment for multifocal motor neuropathy: a randomised, double-blind, multi-center cross-over non-inferiority study vs Kiovig® - The LIME Study. Journal of the Peripheral Nervous System 2019;24(1):56-63. - PMC - PubMed
-
- Leger JM, Hughes R, Merkies ISJ, Nobile-Orazio E, Malyszczak W, Puget S. A European, randomised, double-blind, active comparator-controlled, cross-over, efficacy and safety study of a new 10% ready-to-use liquid human intravenous immunoglobulin (I10E) versus KIOVIG in patients with multifocal motor neuropathy. Journal of the Peripheral Nervous System 2015;20(2):181.
-
- Leger JM, Hughes RAC, Merkies ISJ, Nobile-Orazio E, Malyszczak W, Puget S. A European, randomised, double-blind, cross-over study of a new 10% human intravenous immunoglobulin versus other IVIG in patients with multifocal motor neuropathy - LIME study. Journal of the Peripheral Nervous System 2016;21(3):189.
-
- Leger JM, Hughes RAC, Merkies ISJ, Nobile-Orazio E, Malyszczak W, Puget S. European, randomised, double-blind, crossover study of a new 10% human intravenous immunoglobulin versus other IVIG in patients with multifocal motor neuropathy - Lime Study. Journal of the Peripheral Nervous System 2016;21:77-8.
Pestronk 1995 {published data only}
-
- Pestronk A. Immunosuppressive treatment of motor neuron syndromes. Archives of Neurology 1995;52(3):230-1. - PubMed
Tan 1994 {published data only}
-
- Tan E, Lynn DJ, Amato AA, Kissel JT, Rammohan KW, Sahenk Z, et al. Immunosuppressive treatment of motor neuron syndromes. Attempts to distinguish a treatable disorder. Archives of Neurology 1994;51(2):194-200. - PubMed
Turnbull 2001 {published data only}
-
- Turnbull J. Multifocal motor neuropathy improved by IVIg. Neurology 2001;56(11):1608. - PubMed
Van den Berg‐Vos 2002 {published data only}
-
- Van den Berg-Vos RM, Franssen H, Wokke JH, Van den Berg LH. Multifocal motor neuropathy: long-term clinical and electrophysiological assessment of intravenous immunoglobulin maintenance treatment. Brain 2002;125(Pt 8):1875-86. - PubMed
Yuki 1993 {published data only}
-
- Yuki N, Yamazaki M. Treatment of multifocal motor neuropathy with a high dosage of intravenous immunoglobulin. Muscle & Nerve 1993;16(2):220-1. - PubMed
Additional references
Albers 1985
-
- Albers JW, Donofrio PD, McGonagle TK. Sequential electrodiagnostic abnormalities in acute inflammatory demyelinating polyradiculoneuropathy. Muscle & Nerve 1985;8(6):528-39. - PubMed
Anonymous 2009
Azulay 1997
Beydoun 2000
-
- Beydoun SR, Copeland D. Bilateral phrenic neuropathy as a presenting feature of multifocal motor neuropathy with conduction block. Muscle & Nerve 2000;23(4):556-9. - PubMed
Bouche 1995
Cavaletti 1998
-
- Cavaletti G, Zincone A, Marzorati L, Frattola L, Molteni F, Navalesi P. Rapidly progressive multifocal motor neuropathy with phrenic nerve paralysis: effect of nocturnal assisted ventilation. Journal of Neurology 1998;245(9):613-6. - PubMed
Chaudhry 1993
-
- Chaudhry V, Corse AM, Cornblath DR, Kuncl RW, Drachman DB, Freimer ML, et al. Multifocal motor neuropathy: response to human immune globulin. Annals of Neurology 1993;33(3):237-42. - PubMed
Chaudhry 2010
-
- Chaudhry V, Cornblath DR. An open-label trial of rituximab (Rituxan®) in multifocal motor neuropathy. Journal of the Peripheral Nervous System 2010;15(3):196-201. - PubMed
Cocito 2014
-
- Cocito D, Merola A, Peci E, Mazzeo A, Fazio R, Francia A, et al. Subcutaneous immunoglobulin in CIDP and MMN: a short-term nationwide study. Journal of Neurology 2014;261(11):2159-64. - PubMed
Cohen 1988
-
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates Inc, 1988.
Cornblath 1991
-
- Cornblath DR, Sumner AJ, Daube J, Gilliat RW, Brown WF, Parry GJ, et al. Conduction block in clinical practice. Muscle & Nerve 1991;14(9):869-71. - PubMed
Dalakas 2003
-
- Dalakas MC, Clark WM. Strokes, thromboembolic events, and IVIg. Rare incidents blemish an excellent safety record. Neurology 2003;60(11):1736-7. - PubMed
Deeks 2019
-
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
de Haan 1993
-
- Haan R, Aaronson N, Limburg M, Hewer RL, Crevel H. Measuring quality of life in stroke. Stroke 1993;24(2):320-7. - PubMed
Delfraissy 1985
-
- Delfraissy JF, Tchernia G, Laurian Y, Wallon C, Galanaud P, Dormont J. Suppressor cell function after intravenous gammaglobulin treatment in adult chronic idiopathic thrombocytopenic purpura. British Journal of Haematology 1985;60(2):315-22. - PubMed
Delmont 2006
-
- Delmont E, Azulay JP, Giorgi R, Attarian S, Verschueren A, Uzenot D, et al. Multifocal motor neuropathy with and without conduction block: a single entity? Neurology 2006;67(4):592-6. - PubMed
Duhem 1994
Eijkhout 2002
-
- Eijkhout HW, Aken WG. Blood components, plasma and plasma products. In: Aronsen JK, editors(s). Side Effects of Drugs Annual. 14th edition. Amsterdam: Elsevier, 2002:398-404.
Feldman 1991
-
- Feldman EL, Bromberg MB, Albers JW, Pestronk A. Immunosuppressive treatment in multifocal motor neuropathy. Annals of Neurology 1991;30(3):397-401. - PubMed
Fitzpatrick 2011
-
- Fitzpatrick AM, Mann CA, Barry S, Brennan K, Overell JR, Willison HJ. An open label clinical trial of complement inhibition in multifocal motor neuropathy. Journal of the Peripheral Nervous System 2011;16(2):84-91. - PubMed
Franssen 1997
-
- Franssen H, Vermeulen M, Jennekens FG. Chronic inflammatory neuropathies. In: Emery AEH, editors(s). Diagnostic Criteria for Neuromuscular Disorders. 2nd edition. London: Royal Society of Medicine Press, 1997:53-9.
Gardulf 1995
-
- Gardulf A, Andersen V, Björkander J, Ericson D, Froland SS, Gustafson R, et al. Subcutaneous immunoglobulin replacement in patients with primary antibody deficiencies: safety and costs. Lancet 1995;345(8946):365–9. - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 20 October 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Hausmanowa‐Petrusewicz 1991
-
- Hausmanowa-Petrusewicz I, Rowińska-Marcińska K, Kopeć A. Chronic acquired demyelinating motor neuropathy. Acta Neurologica Scandinavica 1991;84(1):40-5. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2019
-
- Higgins JPT, Eldridge S, Li T, editor(s). Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Higgins 2020
-
- Higgins JPT, Lasserson T, Chandler J, Tovey D, Thomas J, Flemyng E, Churchill R. Methodological Expectations of Cochrane Intervention Reviews. Cochrane: London, Version March 2020.
Hughes 2001
-
- Hughes RAC. 79th ENMC International Workshop: Multifocal motor neuropathy: 14-15 April 2000, Hilversum, The Netherlands. Neuromuscular Disorders 2001;11(3):309-14. - PubMed
Ioannidis 1995
-
- Ioannidis JPA, Cappelleri JC, Lau J, Skolnik PR, Melville B, Chalmers TC, et al. Early or deferred zidovudine therapy in HIV-infected patients without an AIDS-defining illness. Annals of Internal Medicine 1995;122(11):856-66. - PubMed
Kaji 1991
-
- Kaji R, Kimura J. Nerve conduction block. Current Opinion in Neurology and Neurosurgery 1991;4(5):744-8.
Kaji 1992
-
- Kaji R, Shibasaki H, Kimura J. Multifocal demyelinating motor neuropathy: cranial nerve involvement and immunoglobulin therapy. Neurology 1992;42(3):506-9. - PubMed
Kaji 2003
-
- Kaji R. Physiology of conduction block in multifocal motor neuropathy and other demyelinating neuropathies. Muscle & Nerve 2003;27(3):285-96. - PubMed
Kapoor 2020
Katz 1997
-
- Katz JS, Wolfe GI, Bryan WW, Jackson CE, Amato AA, Barohn RJ. Electrophysiologic findings in multifocal motor neuropathy. Neurology 1997;48(3):700-7. - PubMed
Kazatchkine 2001
-
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. New England Journal of Medicine 2001;345(10):747-55. - PubMed
Krarup 1990
-
- Krarup C, Stewart JD, Sumner AJ, Pestronk A, Lipton SA. A syndrome of asymmetric limb weakness with motor conduction block. Neurology 1990;40(1):118-27. - PubMed
Lambrecq 2009
-
- Lambrecq V, Krim E, Rouanet-Larrivière M, Lagueny A. Sensory loss in multifocal motor neuropathy: a clinical and electrophysiological study. Muscle & Nerve 2009;39(2):131-6. - PubMed
Léger 1994
Leung 1987
Lewis 1982
-
- Lewis RA, Sumner AJ, Brown MJ, Asbury AK. Multifocal demyelinating neuropathy with persistent conduction block. Neurology 1982;32(9):958-64. - PubMed
Lievens 2009
-
- Lievens I, Fournier E, Viala K, Maisonobe T, Bouche P, Léger JM. Multifocal motor neuropathy: a retrospective study of sensory nerve conduction velocities in long-term follow-up of 21 patients. Revue Neurologique 2009;165(3):243-8. - PubMed
Macey 1990
-
- Macey MG, Newland AC. CD4 and CD8 subpopulation changes during high dose intravenous immunoglobulin treatment. British Journal of Haematology 1990;76(4):513-20. - PubMed
Martin 2000
-
- Martin TD. Safety and tolerability of intravenous immunoglobulins. In: Said G, editors(s). Treatment of Neurological Disorders With Intravenous Immunoglobulins. London: Martin Duntiz, 2000:181-91.
Martina 1999
McCrone 2003
-
- McCrone P, Chisholm D, Knapp M, Hughes R, Comi G, Dalakas MC, et al. Cost-utility analysis of intravenous immunoglobulin and prednisolone for chronic inflammatory demyelinating polyradiculopathy. European Journal of Neurology 2003;10(6):687-94. - PubMed
Meucci 1997
Moher 2015
Molenaar 1998
-
- Molenaar DSM. Chronic Inflammatory Demyelinating Polyneuropathy: Diagnosis, Treatment, and Outcome Assessment [Thesis]. University of Amsterdam, 1998. [ISBN 90-9012174-9]
Molenaar 1999
-
- Molenaar DSM, Vermeulen M, Visser M, Haan R. Impact of neurological signs and symptoms on functional status in patients with peripheral neuropathies. Neurology 1999;52(1):151-6. - PubMed
Nemni 2003
-
- Nemni R, Santuccio G, Calabrese E, Galardi G, Canal N. Efficacy of cyclosporine treatment in multifocal motor neuropathy. Journal of Neurology 2003;250(9):1118-20. - PubMed
NHS England 2018
-
- NHS England. Updated Commissioning Guidance for the use of therapeutic immunoglobulin (Ig) in immunology, haematology, neurology and infectious diseases in England December 2018. Available at www.england.nhs.uk/wp-content/uploads/2019/03/PSS9-Immunoglobulin-Commis....
Nobile‐Orazio 2001
-
- Nobile-Orazio E. Multifocal motor neuropathy. Journal of Neuroimmunology 2001;115(1-2):4-18. - PubMed
Nobile‐Orazio 2002
-
- Nobile-Orazio E, Cappellari A, Meucci N, Carpo M, Terenghi F, Bersano A, et al. Multifocal motor neuropathy: clinical and immunological features and response to IVIg in relation to the presence and degree of motor conduction block. Journal of Neurology, Neurosurgery and Psychiatry 2002;72(6):761-6. - PMC - PubMed
Nobile‐Orazio 2009
-
- Nobile-Orazio E, Terenghi F, Cocito D, Gallia F, Casellato C. Oral methotrexate as adjunctive therapy in patients with multifocal motor neuropathy on chronic IVIg therapy. Journal of the Peripheral Nervous System 2009;14(3):203-5. - PubMed
Nodera 2006
-
- Nodera H, Bostock H, Izumi Y, Nakamura K, Urushihara R, Sakamoto T, et al. Activity-dependent conduction block in multifocal motor neuropathy: magnetic fatigue test. Neurology 2006;67(2):280-7. - PubMed
Norris 1974
-
- Norris FH Jr, Calanchini PR, Fallat RJ, Pancharis S, Jewett B. Administration of guanidine in amyotrophic lateral sclerosis. Neurology 1974;24(8):721-8. - PubMed
Oh 1994
-
- Oh SJ, Kim DE, Kuruoglu HR. What is the best diagnostic index of conduction block and temporal dispersion? Muscle & Nerve 1994;17(5):489-93. - PubMed
Olney 2003
-
- Olney RK, Lewis RA, Putnam TD, Campellone JVJ. Consensus criteria for the diagnosis of multifocal motor neuropathy. Muscle & Nerve 2003;27(1):117-21. - PubMed
Page 2019
-
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Pakiam 1998
-
- Pakiam AS, Parry GJ. Multifocal motor neuropathy without overt conduction block. Muscle & Nerve 1998;21(2):243-5. - PubMed
Parry 1992
-
- Parry GJ, Sumner AJ. Multifocal motor neuropathy. In: Dyck PJ, Thomas PK, Griffin JW, editors(s). Neurologic Clinics. Peripheral Neuropathy: New Concepts and Treatments. 1st edition. Philadelphia: WB Saunders Company, 1992:671-84. - PubMed
Parry 1993
-
- Parry GJ. Motor neuropathy with multifocal conduction block. Seminars in Neurology 1993;13(3):269-75. - PubMed
Pestronk 1988
-
- Pestronk A, Cornblath DR, Ilyas AA, Baba H, Quarles RH, Griffin JW, et al. A treatable multifocal motor neuropathy with antibodies to GM1 ganglioside. Annals of Neurology 1988;24(1):73-8. - PubMed
Piepers 2007
-
- Piepers S, den Berg-Vos R, Pol WL, Franssen H, Wokke J, den Berg L. Mycophenolate mofetil as adjunctive therapy for MMN patients: a randomized, controlled trial. Brain 2007;130(Pt 8):2004-10. - PubMed
Pierce 2003
-
- Pierce LR, Jain N. Risks associated with the use of intravenous immunoglobulin. Transfusion Medicine Reviews 2003;17(4):241-51. - PubMed
Pringle 1997
-
- Pringle CE, Belden J, Veitch JE, Brown WF. Multifocal motor neuropathy presenting as ophthalmoplegia. Muscle & Nerve 1997;20(3):347-51. - PubMed
Rajabally 2011
-
- Rajabally YA, Kearney DA. Thromboembolic complications of intravenous immunoglobulin therapy in patients with neuropathy: a two-year study. Journal of the Neurological Sciences 2011;308(1878-5883 (Electronic), 0022-510X (Linking), 1-2):124-7. - PubMed
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Rhee 1990
-
- Rhee EK, England JD, Sumner AJ. A computer simulation of conduction block: effects produced by actual conduction block versus interphase cancellation. Annals of Neurology 1990;28(2):146-56. - PubMed
Rothman 1986
-
- Rothman KJ. Modern Epidemiology. Boston: Little, Brown and Company, 1986.
Schünemann 2019a
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Schünemann 2019b
-
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, Akl EA, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (updated July 2019). Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Stiehm 1996
-
- Stiehm ER. Immunoglobulin therapy in primary antibody deficiency and HIV infection. In: Kazatchkine MD, Morell A, editors(s). Intravenous Immunoglobulin: Research and Therapy. 1st edition. Pearl River, NY: Parthenon Publishing, 1996:193-203.
Terenghi 2004
-
- Terenghi F, Cappellari A, Bersano A, Carpo M, Barbieri S, Nobile-Orazio E. How long is IVIg effective in multifocal motor neuropathy? Neurology 2004;62(4):666-8. - PubMed
Umapathi 2015
Van Asseldonk 2003
-
- Van Asseldonk JTH, Van den Berg LH, Van den Berg-Vos RM, Wieneke GH, Wokke JHJ, Franssen H. Demyelination and axonal loss in multifocal motor neuropathy: distribution and relation to weakness. Brain 2003;126(1):186-98. - PubMed
Van Asseldonk 2006
-
- Van Asseldonk JTH, Van den Berg LH, Wieneke GH, Wokke JH, Franssen H. Criteria for conduction block based on computer simulation studies of nerve conduction with human data obtained in the forearm segment of the median nerve. Brain 2006;129(Pt 9):2447-60. - PubMed
Van den Berg 1998
-
- Van den Berg LH, Franssen H, Wokke JH. The long-term effect of intravenous immunoglobulin treatment in multifocal motor neuropathy. Brain 1998;121(Pt 3):421-8. - PubMed
Van den Berg‐Vos 2000a
-
- Van den Berg-Vos RM, Franssen H, Wokke JHJ, Van Es HW, Van den Berg LH. Multifocal motor neuropathy: diagnostic criteria that predict the response to immunoglobulin treatment. Annals of Neurology 2000;48(6):919-26. - PubMed
Van den Berg‐Vos 2000b
-
- Van den Berg-Vos RM, Van den Berg LH, Franssen H, Van Doorn PA, Merkies ISJ, Wokke JHJ. Treatment of multifocal motor neuropathy with interferon beta 1a. Neurology 2000;54(7):1518-21. - PubMed
Van Es 1997
-
- Van Es HW, Van den Berg LH, Franssen H, Witkamp TD, Ramos LM, Notermans NC, et al. Magnetic resonance imaging of the brachial plexus in patients with multifocal motor neuropathy. Neurology 1997;48(5):1218-24. - PubMed
Van Schaik 1994
Van Schaik 1995
-
- Van Schaik IN, Bossuyt PMM, Brand A, Vermeulen M. The diagnostic value of GM1 antibodies in motor neuron disorders and neuropathies: a meta-analysis. Neurology 1995;45(8):1570-7. - PubMed
Van Schaik 2010
-
- Van Schaik IN, Bouche P, Illa I, Léger JM, Van den Bergh P, Cornblath DR, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Journal of the Peripheral Nervous System 2010;15(4):295-301. [DOI: 10.1111/j.1529-8027.2010.00290.x.] [PMID: PMID: 21199100] - DOI - PubMed
Vucic 2004
-
- Vucic S, Black KR, Chong PST, Cros D. Multifocal motor neuropathy. Decrease in conduction blocks and reinnervation with long-term IVIg. Neurology 2004;63(7):1264-9. - PubMed
WHO 1982
Willison 2002
-
- Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain 2002;125(12):2591-625. - PubMed
Wittstock 2003
-
- Wittstock M, Benecke R, Zettl UK. Therapy with intravenous immunoglobulins: complications and side-effects. European Neurology 2003;50(3):172-5. - PubMed
Yu 1999
-
- Yu Z, Lennon VA. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. New England Journal of Medicine 1999;340(3):227-8. - PubMed
Yuki 2011
-
- Yuki N, Watanabe H, Nakajima T, Späth PJ. IVIG blocks complement deposition mediated by anti-GM1 antibodies in multifocal motor neuropathy. Journal of Neurology Neurosurgery and Psychiatry 2011;82(1):87-91. - PubMed
References to other published versions of this review
Van Schaik 2003
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

