Synthesis of C-Oligosaccharides through Versatile C(sp3 )-H Glycosylation of Glycosides
- PMID: 35015329
- PMCID: PMC9306939
- DOI: 10.1002/anie.202114993
Synthesis of C-Oligosaccharides through Versatile C(sp3 )-H Glycosylation of Glycosides
Abstract
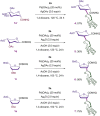
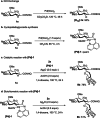
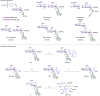
C-oligosaccharides are pharmacologically relevant because they are more hydrolysis-resistant than O-oligosaccharides. Despite indisputable advances, C-oligosaccharides continue to be underdeveloped, likely due to a lack of efficient and selective strategies for the assembly of the interglycosidic C-C linkages. In contrast, we, herein, report a versatile and robust strategy for the synthesis of structurally complex C-oligosaccharides via catalyzed C(sp3 )-H activations. Thus, a wealth of complex interglycosidic (2→1)- and (1→1)-C-oligosaccharides becomes readily available by palladium-catalyzed C(sp3 )-H glycoside glycosylation. The isolation of key palladacycle intermediates and experiments with isotopically-labeled compounds identified a trans-stereoselectivity for the C(sp3 )-H glycosylation. The glycoside C(sp3 )-H activation manifold was likewise exploited for the diversification of furanoses, pyranoses and disaccharides.
Keywords: 2-Deoxyglycosides; C(sp3)−H Activation; C-Disaccharide Synthesis; Palladium Catalysis.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Dwek R. A., Chem. Rev. 1996, 96, 683–720. - PubMed
-
- Simon P. M., Drug Discovery Today 1996, 1, 522–528.
-
- Wen L., Edmunds G., Gibbons C., Zhang J., Gadi M. R., Zhu H., Fang J., Liu X., Kong Y., Wang P. G., Chem. Rev. 2018, 118, 8151–8187. - PubMed
-
- None
-
- Plante O. J., Palmacci E. R., Seeberger P. H., Science 2001, 291, 1523–1527; - PubMed
Publication types
LinkOut - more resources
Full Text Sources

