Angiogenesis depends upon EPHB4-mediated export of collagen IV from vascular endothelial cells
- PMID: 35015735
- PMCID: PMC8876457
- DOI: 10.1172/jci.insight.156928
Angiogenesis depends upon EPHB4-mediated export of collagen IV from vascular endothelial cells
Abstract
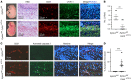
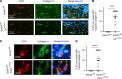
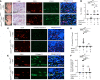
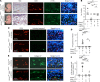
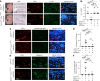
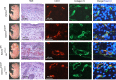
Capillary malformation-arteriovenous malformation (CM-AVM) is a blood vascular anomaly caused by inherited loss-of-function mutations in RASA1 or EPHB4 genes, which encode p120 Ras GTPase-activating protein (p120 RasGAP/RASA1) and Ephrin receptor B4 (EPHB4). However, whether RASA1 and EPHB4 function in the same molecular signaling pathway to regulate the blood vasculature is uncertain. Here, we show that induced endothelial cell-specific (EC-specific) disruption of Ephb4 in mice resulted in accumulation of collagen IV in the EC ER, leading to EC apoptotic death and defective developmental, neonatal, and pathological angiogenesis, as reported previously in induced EC-specific RASA1-deficient mice. Moreover, defects in angiogenic responses in EPHB4-deficient mice could be rescued by drugs that inhibit signaling through the Ras pathway and drugs that promote collagen IV export from the ER. However, EPHB4-mutant mice that expressed a form of EPHB4 that is unable to physically engage RASA1 but retains protein tyrosine kinase activity showed normal angiogenic responses. These findings provide strong evidence that RASA1 and EPHB4 function in the same signaling pathway to protect against the development of CM-AVM independent of physical interaction and have important implications for possible means of treatment of this disease.
Keywords: Angiogenesis; Endothelial cells; Signal transduction; Vascular Biology.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

