Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in children and adolescents in Africa: A randomised, placebo-controlled, multicentre Phase II clinical trial
- PMID: 35015777
- PMCID: PMC8752100
- DOI: 10.1371/journal.pmed.1003865
Safety and immunogenicity of 2-dose heterologous Ad26.ZEBOV, MVA-BN-Filo Ebola vaccination in children and adolescents in Africa: A randomised, placebo-controlled, multicentre Phase II clinical trial
Abstract
Background: Reoccurring Ebola outbreaks in West and Central Africa have led to serious illness and death in thousands of adults and children. The objective of this study was to assess safety, tolerability, and immunogenicity of the heterologous 2-dose Ad26.ZEBOV, MVA-BN-Filo vaccination regimen in adolescents and children in Africa.
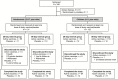
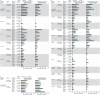
Methods and findings: In this multicentre, randomised, observer-blind, placebo-controlled Phase II study, 131 adolescents (12 to 17 years old) and 132 children (4 to 11 years old) were enrolled from Eastern and Western Africa and randomised 5:1 to receive study vaccines or placebo. Vaccine groups received intramuscular injections of Ad26.ZEBOV (5 × 1010 viral particles) and MVA-BN-Filo (1 × 108 infectious units) 28 or 56 days apart; placebo recipients received saline. Primary outcomes were safety and tolerability. Solicited adverse events (AEs) were recorded until 7 days after each vaccination and serious AEs (SAEs) throughout the study. Secondary and exploratory outcomes were humoral immune responses (binding and neutralising Ebola virus [EBOV] glycoprotein [GP]-specific antibodies), up to 1 year after the first dose. Enrolment began on February 26, 2016, and the date of last participant last visit was November 28, 2018. Of the 263 participants enrolled, 217 (109 adolescents, 108 children) received the 2-dose regimen, and 43 (20 adolescents, 23 children) received 2 placebo doses. Median age was 14.0 (range 11 to 17) and 7.0 (range 4 to 11) years for adolescents and children, respectively. Fifty-four percent of the adolescents and 51% of the children were male. All participants were Africans, and, although there was a slight male preponderance overall, the groups were well balanced. No vaccine-related SAEs were reported; solicited AEs were mostly mild/moderate. Twenty-one days post-MVA-BN-Filo vaccination, binding antibody responses against EBOV GP were observed in 100% of vaccinees (106 adolescents, 104 children). Geometric mean concentrations tended to be higher after the 56-day interval (adolescents 13,532 ELISA units [EU]/mL, children 17,388 EU/mL) than the 28-day interval (adolescents 6,993 EU/mL, children 8,007 EU/mL). Humoral responses persisted at least up to Day 365. A limitation of the study is that the follow-up period was limited to 365 days for the majority of the participants, and so it was not possible to determine whether immune responses persisted beyond this time period. Additionally, formal statistical comparisons were not preplanned but were only performed post hoc.
Conclusions: The heterologous 2-dose vaccination was well tolerated in African adolescents and children with no vaccine-related SAEs. All vaccinees displayed anti-EBOV GP antibodies after the 2-dose regimen, with higher responses in the 56-day interval groups. The frequency of pyrexia after vaccine or placebo was higher in children than in adolescents. These data supported the prophylactic indication against EBOV disease in a paediatric population, as licenced in the EU.
Trial registration: ClinicalTrials.gov NCT02564523.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: ZA, HB, CB, LR, CL and RT report grants from IMI2-2 [Grant Agreement EBOVAC2 (No.115861) from the Innovative Medicines Initiative 2 Joint Undertaking which receives support from the European Union’s Horizon 2020 research and innovation programme] during the conduct of the study. SBS reports grants from Janssen Vaccines & Prevention B.V. during the conduct of the study. MK was a full-time employee of Janssen, Pharmaceutical Companies of Johnson & Johnson at the time of the study. GS, CR, AG, DH, VB, KL, ML and MD were full-time employees of Janssen, Pharmaceutical Companies of Johnson & Johnson at the time of the study, and may own shares in Janssen, Pharmaceutical Companies of Johnson & Johnson. All other authors have nothing to disclose.
Figures

References
-
- World Health Organization. Situation Report: Ebola Virus Disease. 2016 Jun 10. [cited 2021 Mar 25]. https://apps.who.int/iris/bitstream/handle/10665/208883/ebolasitrep_10Ju....
-
- World Health Organization. Ebola virus disease: Democratic Republic of the Congo. External Situation Report 96. 2020 Jun 9. [cited 2021 Mar 25]. https://apps.who.int/iris/bitstream/handle/10665/332320/SITREP_EVD_DRC_2....
-
- Henao-Restrepo AM, Camacho A, Longini IM, Watson CH, Edmunds WJ, Egger M, et al.. Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). Lancet. 2017;389(10068):505–18. doi: 10.1016/S0140-6736(16)32621-6 - DOI - PMC - PubMed
-
- World Health Organization. Preliminary results on the efficacy of rVSV-ZEBOV-GP Ebola vaccine using the ring vaccination strategy in the control of an Ebola outbreak in the Democratic Republic of the Congo: an example of integration of research into epidemic response. [cited 2021 Mar 25]. https://www.who.int/csr/resources/publications/ebola/ebola-ring-vaccinat....
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

