Nucleic Acid Point-of-Care Testing to Improve Diagnostic Preparedness
- PMID: 35015842
- PMCID: PMC9464067
- DOI: 10.1093/cid/ciac013
Nucleic Acid Point-of-Care Testing to Improve Diagnostic Preparedness
Abstract
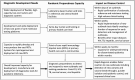
Testing programs for severe acute respiratory syndrome coronavirus 2 have relied on high-throughput polymerase chain reaction laboratory tests and rapid antigen assays to meet diagnostic needs. Both technologies are essential; however, issues of cost, accessibility, manufacturing delays, and performance have limited their use in low-resource settings and contributed to the global inequity in coronavirus disease 2019 testing. Emerging low-cost, multidisease point-of-care nucleic acid tests may address these limitations and strengthen pandemic preparedness, especially within primary healthcare where most cases of disease first present. Widespread deployment of these novel technologies will also help close long-standing test access gaps for other diseases, including tuberculosis, human immunodeficiency virus, cervical cancer, viral hepatitis, and sexually transmitted infections. We propose a more optimized testing framework based on greater use of point-of-care nucleic acid tests together with rapid immunologic assays and high-throughput laboratory molecular tests to improve the diagnosis of priority endemic and epidemic diseases, as well as strengthen the overall delivery of primary healthcare services.
Keywords: COVID-19; diagnosis; nucleic acid test; point-of-care; primary healthcare.
© The Author(s) 2022. Published by Oxford University Press for the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. I. J. reports grants to his institution from the World Health Organization and the European and Developing Countries Clinical Trial Partnership. T. F. P. reports no potential conflicts of interest. Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures

References
-
- Hannay E, Sands P.. One year into the Covid-19 pandemic, testing is as vital as ever. 2021. Available at: https://www.telegraph.co.uk/global-health/science-and-disease/one-year-c.... Accessed 17 August 2021.
-
- World Health Organization . Public health criteria to adjust public health and social measures in the context of COVID-19: annex to considerations in adjusting public health and social measures in the context of COVID-19, 12 May 2020. Available at: https://apps.who.int/iris/handle/10665/332073. Accessed 24 August 2021.
-
- Nkengasong J. Let Africa into the market for COVID-19 diagnostics. Nature 2020; 580:565. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

