Point-of-care testing in Paediatric settings in the UK and Ireland: a cross-sectional study
- PMID: 35016622
- PMCID: PMC8753865
- DOI: 10.1186/s12873-021-00556-7
Point-of-care testing in Paediatric settings in the UK and Ireland: a cross-sectional study
Abstract
Background: Point-of-care testing (POCT) is diagnostic testing performed at or near to the site of the patient. Understanding the current capacity, and scope, of POCT in this setting is essential in order to respond to new research evidence which may lead to wide implementation.
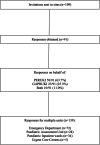
Methods: A cross-sectional online survey study of POCT use was conducted between 6th January and 2nd February 2020 on behalf of two United Kingdom (UK) and Ireland-based paediatric research networks (Paediatric Emergency Research UK and Ireland, and General and Adolescent Paediatric Research UK and Ireland).
Results: In total 91/109 (83.5%) sites responded, with some respondents providing details for multiple units on their site based on network membership (139 units in total). The most commonly performed POCT were blood sugar (137/139; 98.6%), urinalysis (134/139; 96.4%) and blood gas analysis (132/139; 95%). The use of POCT for Influenza/Respiratory Syncytial Virus (RSV) (45/139; 32.4%, 41/139; 29.5%), C-Reactive Protein (CRP) (13/139; 9.4%), Procalcitonin (PCT) (2/139; 1.4%) and Group A Streptococcus (5/139; 3.6%) and was relatively low. Obstacles to the introduction of new POCT included resources and infrastructure to support test performance and quality assurance.
Conclusion: This survey demonstrates significant consensus in POCT practice in the UK and Ireland but highlights specific inequity in newer biomarkers, some which do not have support from national guidance. A clear strategy to overcome the key obstacles of funding, evidence base, and standardising variation will be essential if there is a drive toward increasing implementation of POCT.
Keywords: Data collection; Health services research; Molecular biology; Technology.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- Lee-Lewandrowski E, Corboy D, Lewandrowski K, Sinclair J, McDermot S, Benzer TI. Implementation of a point-of-care satellite laboratory in the emergency department of an academic medical center: impact on test turnaround time and patient emergency department length of stay. Arch Pathol Lab Med. 2003;127(4):456–460. doi: 10.5858/2003-127-0456-IOAPSL. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous