Total neoadjuvant therapy for pancreatic adenocarcinoma increases probability for a complete pathologic response
- PMID: 35016837
- PMCID: PMC9233019
- DOI: 10.1016/j.ejso.2021.12.473
Total neoadjuvant therapy for pancreatic adenocarcinoma increases probability for a complete pathologic response
Abstract
Background: Multiple neoadjuvant therapy protocols have been proposed in the treatment of pancreatic adenocarcinoma, including chemotherapy (CT), chemoradiation (CRT), and total neoadjuvant therapy (TNT), defined as a CT plus CRT. A pathologic complete response (pCR) can be achieved in a minority of cases. We hypothesize that TNT is more likely to confer pCR than other neoadjuvant therapies, which may improve overall survival (OS).
Methods: A retrospective review of the National Cancer Database (NCDB) from 2006 to 2016 was performed, identifying patients who underwent any neoadjuvant therapy followed by definitive pancreatic resection for locally advanced or borderline resectable pancreatic adenocarcinoma. A pathologic complete response was defined as down-staging from any clinical stage to pathologic stage 0.
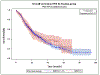
Results: A total of 5402 patients who received neoadjuvant therapy followed by resection were identified. 177 patients (3.3%) achieved a pCR. Of the patients who achieved a pCR, 57 received CT, 41 CRT and 79 received TNT. On multivariate analysis, TNT was more likely to confer a pCR than CRT (OR 1.67, CI 1.13-2.46, p = 0.0103) or CT (OR 2.61, CI 1.83-3.71, p < 0.0001). Patients who achieved pCR had a significantly higher OS, with median survival of 64.9 months, compared to 21.6 months in patients who did not achieve pCR (p < 0.0001).
Conclusion: TNT may be more likely to achieve a pCR than CT or CRT. Patients who achieve a pCR have a significant OS benefit as compared to those who have residual disease. TNT should be considered for patients requiring neoadjuvant therapy, as it may increase the likelihood of achieving a pCR, thus potentially improving OS.
Keywords: Chemoradiation; Chemotherapy; Pancreas; Radiation; TNT; Whipple.
Copyright © 2021. Published by Elsevier Ltd.
Figures
References
-
- Conroy T, Hammel P, Hebbar M, et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. The New England journal of medicine. Dec 20 2018;379(25):2395–2406. - PubMed
-
- Rose JB, Rocha FG, Alseidi A, et al. Extended neoadjuvant chemotherapy for borderline resectable pancreatic cancer demonstrates promising postoperative outcomes and survival. Annals of surgical oncology. May 2014;21(5):1530–1537. - PubMed
-
- Vidri RJ, Vogt AO, Macgillivray DC, Bristol IJ, Fitzgerald TL. Better Defining the Role of Total Neoadjuvant Radiation: Changing Paradigms in Locally Advanced Pancreatic Cancer. Annals of surgical oncology. Oct 2019;26(11):3701–3708. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

