Cross reactivity of spike glycoprotein induced antibody against Delta and Omicron variants before and after third SARS-CoV-2 vaccine dose in healthy and immunocompromised individuals
- PMID: 35016901
- PMCID: PMC8743815
- DOI: 10.1016/j.jinf.2022.01.002
Cross reactivity of spike glycoprotein induced antibody against Delta and Omicron variants before and after third SARS-CoV-2 vaccine dose in healthy and immunocompromised individuals
Keywords: COVID-19; Chronic kidney disease; Cross reactivity; Delta; Haemodialysis; Omicron; SARS-CoV-2; Variants of Concern; antibody response; vaccination.
Figures
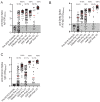
References
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. Jan. - PMC - PubMed
-
- England P.H. SARS-CoV-2 variants of concern and variants under investigation in England. Sage. 2021;(April).
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

