Systemic high-dose dexamethasone treatment may modulate the efficacy of intratumoral viral oncolytic immunotherapy in glioblastoma models
- PMID: 35017150
- PMCID: PMC8753448
- DOI: 10.1136/jitc-2021-003368
Systemic high-dose dexamethasone treatment may modulate the efficacy of intratumoral viral oncolytic immunotherapy in glioblastoma models
Abstract
Background: Intratumoral viral oncolytic immunotherapy is a promising new approach for the treatment of a variety of solid cancers. CAN-2409 is a replication-deficient adenovirus that delivers herpes simplex virus thymidine kinase to cancer cells, resulting in local conversion of ganciclovir or valacyclovir into a toxic metabolite. This leads to highly immunogenic cell death, followed by a local immune response against a variety of cancer neoantigens and, next, a systemic immune response against the injected tumor and uninjected distant metastases. CAN-2409 treatment has shown promising results in clinical studies in glioblastoma (GBM). Patients with GBM are usually given the corticosteroid dexamethasone to manage edema. Previous work has suggested that concurrent dexamethasone therapy may have a negative effect in patients treated with immune checkpoint inhibitors in patients with GBM. However, the effects of dexamethasone on the efficacy of CAN-2409 treatment have not been explored.
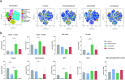
Methods: In vitro experiments included cell viability and neurosphere T-cell killing assays. Effects of dexamethasone on CAN-2409 in vivo were examined using a syngeneic murine GBM model; survival was assessed according to Kaplan-Meier; analyses of tumor-infiltrating lymphocytes were performed with mass cytometry (CyTOF - cytometry by time-of-flight). Data were analyzed using a general linear model, with one-way analysis of variance followed by Dunnett's multiple comparison test, Kruskal-Wallis test, Dunn's multiple comparison test or statistical significance analysis of microarrays.
Results: In a mouse model of GBM, we found that high doses of dexamethasone combined with CAN-2409 led to significantly reduced median survival (29.0 days) compared with CAN-2409 treatment alone (39.5 days). CyTOF analyses of tumor-infiltrating immune cells demonstrated potent immune stimulation induced by CAN-2409 treatment. These effects were diminished when high-dose dexamethasone was used. Functional immune cell characterization suggested increased immune cell exhaustion and tumor promoting profiles after dexamethasone treatment.
Conclusion: Our data suggest that concurrent high-dose dexamethasone treatment may impair the efficacy of oncolytic viral immunotherapy of GBM, supporting the notion that dexamethasone use should be balanced between symptom control and impact on the therapeutic outcome.
Keywords: brain neoplasms; oncolytic virotherapy; translational medical research; tumor microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
