Using Machine Learning to Identify Metabolomic Signatures of Pediatric Chronic Kidney Disease Etiology
- PMID: 35017168
- PMCID: PMC8819986
- DOI: 10.1681/ASN.2021040538
Using Machine Learning to Identify Metabolomic Signatures of Pediatric Chronic Kidney Disease Etiology
Abstract
Background: Untargeted plasma metabolomic profiling combined with machine learning (ML) may lead to discovery of metabolic profiles that inform our understanding of pediatric CKD causes. We sought to identify metabolomic signatures in pediatric CKD based on diagnosis: FSGS, obstructive uropathy (OU), aplasia/dysplasia/hypoplasia (A/D/H), and reflux nephropathy (RN).
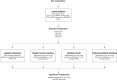
Methods: Untargeted metabolomic quantification (GC-MS/LC-MS, Metabolon) was performed on plasma from 702 Chronic Kidney Disease in Children study participants (n: FSGS=63, OU=122, A/D/H=109, and RN=86). Lasso regression was used for feature selection, adjusting for clinical covariates. Four methods were then applied to stratify significance: logistic regression, support vector machine, random forest, and extreme gradient boosting. ML training was performed on 80% total cohort subsets and validated on 20% holdout subsets. Important features were selected based on being significant in at least two of the four modeling approaches. We additionally performed pathway enrichment analysis to identify metabolic subpathways associated with CKD cause.
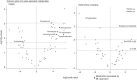
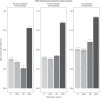
Results: ML models were evaluated on holdout subsets with receiver-operator and precision-recall area-under-the-curve, F1 score, and Matthews correlation coefficient. ML models outperformed no-skill prediction. Metabolomic profiles were identified based on cause. FSGS was associated with the sphingomyelin-ceramide axis. FSGS was also associated with individual plasmalogen metabolites and the subpathway. OU was associated with gut microbiome-derived histidine metabolites.
Conclusion: ML models identified metabolomic signatures based on CKD cause. Using ML techniques in conjunction with traditional biostatistics, we demonstrated that sphingomyelin-ceramide and plasmalogen dysmetabolism are associated with FSGS and that gut microbiome-derived histidine metabolites are associated with OU.
Keywords: chronic kidney disease; machine learning; machine learning collection; metabolomics; pediatric nephrology.
Copyright © 2022 by the American Society of Nephrology.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

