Bacterial biofilms predominate in both acute and chronic human lung infections
- PMID: 35017313
- PMCID: PMC9510407
- DOI: 10.1136/thoraxjnl-2021-217576
Bacterial biofilms predominate in both acute and chronic human lung infections
Abstract
Background: A basic paradigm of human infection is that acute bacterial disease is caused by fast growing planktonic bacteria while chronic infections are caused by slow-growing, aggregated bacteria, a phenomenon known as a biofilm. For lung infections, this paradigm has been thought to be supported by observations of how bacteria proliferate in well-established growth media in the laboratory-the gold standard of microbiology.
Objective: To investigate the bacterial architecture in sputum from patients with acute and chronic lung infections.
Methods: Advanced imaging technology was used for quantification and direct comparison of infection types on fresh sputum samples, thereby directly testing the acute versus chronic paradigm.
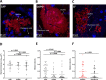
Results: In this study, we compared the bacterial lifestyle (planktonic or biofilm), growth rate and inflammatory response of bacteria in freshly collected sputum (n=43) from patient groups presenting with acute or chronic lung infections. We found that both acute and chronic lung infections are dominated by biofilms (aggregates of bacteria within an extracellular matrix), although planktonic cells were observed in both sample types. Bacteria grew faster in sputum from acute infections, but these fast-growing bacteria were enriched in biofilms similar to the architecture thought to be reserved for chronic infections. Cellular inflammation in the lungs was also similar across patient groups, but systemic inflammatory markers were only elevated in acute infections.
Conclusions: Our findings indicate that the current paradigm of equating planktonic with acute and biofilm with chronic infection needs to be revisited as the difference lies primarily in metabolic rates, not bacterial architecture.
Keywords: bacterial infection; cystic fibrosis; pneumonia; respiratory infection.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
