Effective Menin inhibitor-based combinations against AML with MLL rearrangement or NPM1 mutation (NPM1c)
- PMID: 35017466
- PMCID: PMC8752621
- DOI: 10.1038/s41408-021-00603-3
Effective Menin inhibitor-based combinations against AML with MLL rearrangement or NPM1 mutation (NPM1c)
Abstract
Treatment with Menin inhibitor (MI) disrupts the interaction between Menin and MLL1 or MLL1-fusion protein (FP), inhibits HOXA9/MEIS1, induces differentiation and loss of survival of AML harboring MLL1 re-arrangement (r) and FP, or expressing mutant (mt)-NPM1. Following MI treatment, although clinical responses are common, the majority of patients with AML with MLL1-r or mt-NPM1 succumb to their disease. Pre-clinical studies presented here demonstrate that genetic knockout or degradation of Menin or treatment with the MI SNDX-50469 reduces MLL1/MLL1-FP targets, associated with MI-induced differentiation and loss of viability. MI treatment also attenuates BCL2 and CDK6 levels. Co-treatment with SNDX-50469 and BCL2 inhibitor (venetoclax), or CDK6 inhibitor (abemaciclib) induces synergistic lethality in cell lines and patient-derived AML cells harboring MLL1-r or mtNPM1. Combined therapy with SNDX-5613 and venetoclax exerts superior in vivo efficacy in a cell line or PD AML cell xenografts harboring MLL1-r or mt-NPM1. Synergy with the MI-based combinations is preserved against MLL1-r AML cells expressing FLT3 mutation, also CRISPR-edited to introduce mtTP53. These findings highlight the promise of clinically testing these MI-based combinations against AML harboring MLL1-r or mtNPM1.
© 2022. The Author(s).
Conflict of interest statement
Gerard M. McGeehan is an employee of Syndax Pharmaceuticals. Benjamin L. Ebert has received research funding from Celgene, Deerfield, Novartis, and Calico; and he is a member of the scientific advisory board and shareholder for Neomorph Therapeutics, Skyhawk Therapeutics, and Exo Therapeutics, none of which are directly related to the content of this paper. The remaining authors declare no competing interests.
Figures

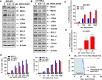


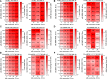
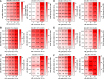

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

