Diacylglyceryl-N,N,N-trimethylhomoserine-dependent lipid remodeling in a green alga, Chlorella kessleri
- PMID: 35017659
- PMCID: PMC8752610
- DOI: 10.1038/s42003-021-02927-z
Diacylglyceryl-N,N,N-trimethylhomoserine-dependent lipid remodeling in a green alga, Chlorella kessleri
Abstract
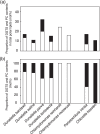
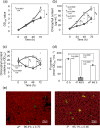
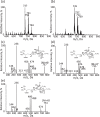
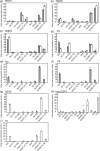
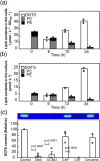
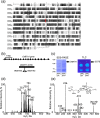
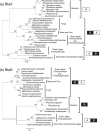
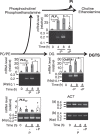
Membrane lipid remodeling contributes to the environmental acclimation of plants. In the green lineage, a betaine lipid, diacylglyceryl-N,N,N-trimethylhomoserine (DGTS), is included exclusively among green algae and nonflowering plants. Here, we show that the green alga Chlorella kessleri synthesizes DGTS under phosphorus-deficient conditions through the eukaryotic pathway via the ER. Simultaneously, phosphatidylcholine and phosphatidylethanolamine, which are similar to DGTS in their zwitterionic properties, are almost completely degraded to release 18.1% cellular phosphorus, and to provide diacylglycerol moieties for a part of DGTS synthesis. This lipid remodeling system that substitutes DGTS for extrachloroplast phospholipids to lower the P-quota operates through the expression induction of the BTA1 gene. Investigation of this lipid remodeling system is necessary in a wide range of lower green plants for a comprehensive understanding of their phosphorus deficiency acclimation strategies.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Nakamura Y. Phosphate starvation and membrane lipid remodeling in seed plants. Prog. Lipid Res. 2013;52:43–50. - PubMed
-
- Sato N. Roles of the acidic lipids sulfoquinovosyl diacylglycerol and phosphatidylglycerol in photosynthesis: their specificity and evolution. J. Plant Res. 2004;117:495–505. - PubMed
-
- Brown AE, Elovson J. Isolation and characterization of a novel lipid, 1(3),2-diacylglyceryl-(3)-O-4′-(N,N,N-trimethyl)homoserine, from Ochromonas danica. Biochemistry. 1974;13:3476–3482. - PubMed
-
- Künzler K, Eichenberger W. Betaine lipids and zwitterionic phospholipids in plants and fungi. Phytochemistry. 1997;46:883–892. - PubMed
-
- Sato N, Furuya M. Distribution of diacylglyceryltrimethylhomoserine in selected species of vascular plants. Phytochemistry. 1984;23:1625–1627.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

