Retinal non-perfusion in diabetic retinopathy
- PMID: 35017700
- PMCID: PMC8807828
- DOI: 10.1038/s41433-021-01649-0
Retinal non-perfusion in diabetic retinopathy
Abstract
Diabetic retinopathy is a major cause of vision loss worldwide and areas of retinal non-perfusion (RNP) are a key pathologic feature of the vascular component of diabetic retinopathy. While there is a need for a more complete understanding of the natural history of RNP development and progression, overall, increasing RNP has been closely linked with worsening DR severity. Both traditional and novel approaches to quantitative image assessment are being explored to advance our understanding of the vascular, physiologic and functional changes associated with progressive RNP. Retinal ischemia secondary to RNP leads to tissue hypoxia and changes in the expression of a host of signalling molecules. Current anti-vascular endothelial growth factor and steroid pharmaceutical agents appear to be unable to reperuse areas of RNP, but may be able to slow the progressive longitudinal accumulation of RNP with regular retreatments. There remains a tremendous unmet need for pharmacotherapies that can slow RNP progression and ultimately reperfuse areas of the non-perfused retina. Towards this end, novel targets including the semaphorin family are being investigated.
糖尿病视网膜病变中的视网膜无灌注
摘要
糖尿病视网膜病变 (DR) 是全球导致视力下降的主要原因, 视网膜无灌注区 (RNP) 是DR血管病变的重要病理特征。在需要更全面地了解RNP发生发展的自然历史的同时, 已发现RNP的增加与DR严重程度的恶化密切相关。传统和创新性的图像定量评估方法正在探索中以提高对进行性RNP相关血管、生理和功能变化的认识。视网膜缺血继发于RNP并导致组织缺氧和一系列信号分子表达的改变。目前的抗血管内皮生长因子和类固醇药物似乎不能对RNP区域进行再灌注, 但可通过定期治疗来减缓RNP的进行性纵向发展。目前对于能够减缓RNP进展并最终使RNP实现再灌注的药物仍有巨大的需求未得到满足。为此, 包括semaphorin蛋白家族在内的新靶点正在研究中。
© 2021. The Author(s), under exclusive licence to The Royal College of Ophthalmologists.
Conflict of interest statement
The authors report the following conflicts of interest: C.C.W: consultant: Adverum, Aerie Pharmaceuticals, Allergan, Apellis, Arctic Vision, Arrowhead Pharmaceuticals, Bausch + Lomb, Bayer, Bionic Vision Technologies, Chengdu Kanghong Biotechnologies, Clearside Biomedical, EyePoint Pharmaceuticals, Genentech, Gyroscope, IVERIC Bio, Kato Pharmaceuticals, Kodiak Sciences, Long Bridge Medical, NGM Biopharmaceuticals, Novartis, OccuRx, Ocular Therapeutix, ONL Therapeutics, Opthea Limited, Oxurion, Palatin, PolyPhotonix, RecensMedical, Regeneron, RegenXBio, Roche, SAI MedPartners, Takeda, Verana Health; research support: Adverum, Aerie Pharmaceuticals, Aldeyra, Alimera Sciences, Allergan, Amgen, Apellis, Asclepix, Bayer, Boehringer Ingelheim, Chengdu Kanghong Biotechnology, Clearside Biomedical, Gemini, Genentech, Graybug Vision, Gyroscope, IONIS Pharmaceutical, iRENIX, IVERIC bio, Kodiak Sciences, LMRI, Neurotech Pharmaceuticals, NGM Biopharmaceuticals, Novartis, Oxurion, RecensMedical, Regeneron, RegenXBio, Roche, SamChunDang Pharm, Taiwan Liposome Company, Xbrane BioPharma; ownership/stock: ONL Therapeutics, PolyPhotonix, RecensMedical, Visgenx. H.J.Y: No conflicts of interest. R.L.A: consultant: Allergan, Alimera, Amgen, Bausch & Lomb, Ocular Therapeutix, Iridex, Novartis, Regeneron, RegenXbio, Santen, Genentech, and Eyepoint; stock: Novartis, Regeneron, Replenish. J.P.E:
Figures
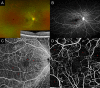
References
-
- de Carlo TE, Chin AT, Bonini Filho MA, Adhi M, Branchini L, Salz DA, et al. Detection of microvascular changes in eyes of patients with diabetes but not clinical diabetic retinopathy using optical coherence tomography angiography. Retina. 2015;35:2364–70. doi: 10.1097/IAE.0000000000000882. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

