Reproxalap Improves Signs and Symptoms of Allergic Conjunctivitis in an Allergen Chamber: A Real-World Model of Allergen Exposure
- PMID: 35018093
- PMCID: PMC8742616
- DOI: 10.2147/OPTH.S345324
Reproxalap Improves Signs and Symptoms of Allergic Conjunctivitis in an Allergen Chamber: A Real-World Model of Allergen Exposure
Abstract
Purpose: To assess the prophylactic and treatment activity of reproxalap, a novel reactive aldehyde species inhibitor, in a real-world model of allergen exposure.
Methods: In a randomized, double-masked, vehicle-controlled, crossover Phase 2 trial, 70 adult patients with ≥2 years of moderate to severe allergic conjunctivitis history, a positive skin test to ragweed pollen, and allergen chamber-induced ocular itching and redness scores of ≥2.5 and ≥2 (both scales range from 0 to 4), respectively, were randomized 1:1:1 to one of three sequences: 0.25% reproxalap, 0.5% reproxalap, and placebo; 0.5% reproxalap, placebo, and 0.25% reproxalap; or placebo, 0.25% reproxalap, and 0.5% reproxalap. Symptoms and conjunctival redness were assessed over 3.5 hours in an allergen chamber of aerosolized ragweed pollen (3500 grains/m3). Test article was administered bilaterally just before chamber entry and at 90 minutes after chamber entry.
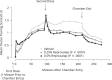
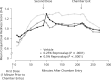
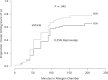
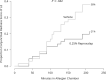
Results: Reproxalap was safe and well tolerated; 66 of 70 enrolled patients completed all visits. Relative to vehicle, both concentrations of reproxalap demonstrated statistically significant and clinically relevant improvements in ocular itching, tearing, and redness over the duration of exposure in the chamber (P < 0.001 for all assessments). Prophylactic and treatment activity of drug were demonstrated.
Conclusion: In an allergen chamber, reproxalap, a novel reactive aldehyde species inhibitor, was statistically superior to vehicle across the typical symptoms and signs of allergic conjunctivitis. These data are among the first rigorous clinical results demonstrating drug improvement in allergic conjunctivitis in an allergen chamber, a real-world model of allergen exposure.
Keywords: RASP inhibitor; allergen chamber; allergic conjunctivitis; inflammation; reproxalap.
© 2022 Clark et al.
Conflict of interest statement
D. Clark reports employment with and stock ownership in Aldeyra Therapeutics. P. Karpecki reports consulting for Aerie Pharmaceuticals, Akorn, Alcon Labs, Aldeyra Therapeutics, Allergan/AbbVie, Allysta, Aurinia, Azura Pharmaceuticals, Bausch & Lomb, BioTissue, BlephEx, Bruder Healthcare, Cambium, Dompe, Eyedetec, EyeGate, Eyevance, Imprimis, Kala Pharmaceuticals, Mallinckrodt, Novartis, Oasis Medical, Oyster Point, Regener-Eyes, ScienceBased Health, Sight Sciences, Silk Technologies, Sun Pharmaceutical Industries, Surface Pharmaceuticals, Tarsus Pharmaceuticals, Visant Medical, and Vital Tears. A. M. Salapatek reports employment with Cliantha Research. J.D. Sheppard reports consulting for Allergan, AbbVie, Alcon, Aldeyra Therapeutics, Bausch & Lomb, NovaBay, Novartis, Fidia, Clarios, Visus, Topivert, Noveome, Oyster Point, Santen, Sun Pharmaceutical Industries, Eyevance, ScienceBased Health, and Quidel; and ownership interest in Noveome, Eyedetec, Oyster Point, RPS, TearLab, EyeGate, EyeRx, Clarios, and CVP Partners. T. C. Brady reports employment with and patent interests and stock ownership in Aldeyra Therapeutics and stock ownership in F-star Therapeutics and Evoke Pharma. The authors report no other conflicts of interest in this work.
Figures

Similar articles
-
Reproxalap in patients with seasonal allergic conjunctivitis: a systematic review and meta-analysis.J Ophthalmic Inflamm Infect. 2025 Apr 28;15(1):39. doi: 10.1186/s12348-025-00497-3. J Ophthalmic Inflamm Infect. 2025. PMID: 40295385 Free PMC article. Review.
-
Reproxalap Activity and Estimation of Clinically Relevant Thresholds for Ocular Itching and Redness in a Randomized Allergic Conjunctivitis Field Trial.Ophthalmol Ther. 2022 Aug;11(4):1449-1461. doi: 10.1007/s40123-022-00520-z. Epub 2022 May 18. Ophthalmol Ther. 2022. PMID: 35585427 Free PMC article.
-
The Phase 3 INVIGORATE Trial of Reproxalap in Patients with Seasonal Allergic Conjunctivitis.Clin Ophthalmol. 2023 Dec 13;17:3867-3875. doi: 10.2147/OPTH.S441009. eCollection 2023. Clin Ophthalmol. 2023. PMID: 38105911 Free PMC article. Clinical Trial.
-
Clinically Relevant Activity of the Novel RASP Inhibitor Reproxalap in Allergic Conjunctivitis: The Phase 3 ALLEVIATE Trial.Am J Ophthalmol. 2021 Oct;230:60-67. doi: 10.1016/j.ajo.2021.04.023. Epub 2021 May 1. Am J Ophthalmol. 2021. PMID: 33945820 Clinical Trial.
-
[Interest of environmental exposure chambers in the evaluation of allergic conjunctivitis].J Fr Ophtalmol. 2020 Nov;43(9):920-928. doi: 10.1016/j.jfo.2020.07.001. Epub 2020 Sep 29. J Fr Ophtalmol. 2020. PMID: 33004194 Review. French.
Cited by
-
Therapeutic Targets in Allergic Conjunctivitis.Pharmaceuticals (Basel). 2022 Apr 28;15(5):547. doi: 10.3390/ph15050547. Pharmaceuticals (Basel). 2022. PMID: 35631374 Free PMC article. Review.
-
Reproxalap in patients with seasonal allergic conjunctivitis: a systematic review and meta-analysis.J Ophthalmic Inflamm Infect. 2025 Apr 28;15(1):39. doi: 10.1186/s12348-025-00497-3. J Ophthalmic Inflamm Infect. 2025. PMID: 40295385 Free PMC article. Review.
-
A Modern Approach to Clinical Outcome Assessment in Allergy Management: Advantages of Allergen Exposure Chambers.J Clin Med. 2024 Nov 29;13(23):7268. doi: 10.3390/jcm13237268. J Clin Med. 2024. PMID: 39685727 Free PMC article. Review.
-
Reproxalap Activity and Estimation of Clinically Relevant Thresholds for Ocular Itching and Redness in a Randomized Allergic Conjunctivitis Field Trial.Ophthalmol Ther. 2022 Aug;11(4):1449-1461. doi: 10.1007/s40123-022-00520-z. Epub 2022 May 18. Ophthalmol Ther. 2022. PMID: 35585427 Free PMC article.
-
The Phase 3 INVIGORATE Trial of Reproxalap in Patients with Seasonal Allergic Conjunctivitis.Clin Ophthalmol. 2023 Dec 13;17:3867-3875. doi: 10.2147/OPTH.S441009. eCollection 2023. Clin Ophthalmol. 2023. PMID: 38105911 Free PMC article. Clinical Trial.
References
-
- Mandell KJ, Clark D, Chu DS, Foster CS, Sheppard J, Brady TC. Randomized phase 2 trial of reproxalap, a novel reactive aldehyde species inhibitor, in patients with noninfectious anterior uveitis: model for corticosteroid replacement. J Ocul Pharmacol Ther. 2020;36(10):732–739. doi:10.1089/jop.2020.0056 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

