Diaphragm Ultrasound is an Imaging Biomarker that Distinguishes Exacerbation Status from Stable Chronic Obstructive Pulmonary Disease
- PMID: 35018095
- PMCID: PMC8742578
- DOI: 10.2147/COPD.S341484
Diaphragm Ultrasound is an Imaging Biomarker that Distinguishes Exacerbation Status from Stable Chronic Obstructive Pulmonary Disease
Abstract
Background: Evaluating the diaphragm muscle in chronic obstructive pulmonary disease (COPD) is important. However, the role of diaphragm ultrasound (DUS) in distinguishing the exacerbation status of COPD (AECOPD) is not fully understood. We set this study to evaluate the role of DUS as a biomarker for distinguishing the AECOPD.
Methods: COPD patients who underwent DUS were enrolled between March 2020 and November 2020. The diaphragm thickening fraction (TFmax) and diaphragm excursion (DEmax) during maximal deep breathing were measured. Patients were divided into exacerbation and stable groups. Demographics, lung function, and DUS findings were compared between the two groups. Receiver operating characteristic curve and univariate/multivariate logistic regression analyses were performed.
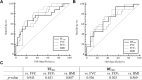
Results: Fifty-five patients were enrolled. The exacerbation group had a lower body mass index (BMI) (20.9 vs 24.2, p = 0.003), lower TFmax (94.8 ± 8.2% vs 158.4 ± 83.5%, p = 0.010), and lower DEmax (30.8 ± 11.1 mm vs 40.5 ± 12.5 mm, p = 0.007) compared to stable group. The areas under the TFmax (0.745) and DEmax (0.721) curves indicated fair results for distinguishing AECOPD. The patients were divided into low and high TFmax and DEmax groups based on calculated cut-off values. Low TFmax (odds ratio [OR] 8.40; 95% confidence interval [CI] 1.55-45.56) and low DEmax (OR 11.51; 95% CI 1.15-115.56) were associated with AECOPD after adjusting for age, sex, BMI, and lung functions.
Conclusion: DUS showed the possibility of an imaging biomarker distinguishing AECOPD from stable status.
Keywords: COPD; biomarker; diaphragm; exacerbation; ultrasound.
© 2022 An et al.
Conflict of interest statement
Prof. Dr. Chin Kook Rhee reports personal fees from MSD, personal fees from AstraZeneca, personal fees from GSK, personal fees from Novartis, personal fees from Takeda, personal fees from Mundipharma, personal fees from Boehringer-Ingelheim, personal fees from Teva, personal fees from Sanofi, personal fees from Bayer, outside the submitted work. The authors have no other conflicts of interest to declare.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

