Immunity to SARS-CoV-2 up to 15 months after infection
- PMID: 35018336
- PMCID: PMC8736281
- DOI: 10.1016/j.isci.2022.103743
Immunity to SARS-CoV-2 up to 15 months after infection
Abstract
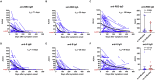
Information concerning the longevity of immunity to SARS-CoV-2 following natural infection may have considerable implications for durability of immunity induced by vaccines. Here, we monitored the SARS-CoV-2 specific immune response in COVID-19 patients followed up to 15 months after symptoms onset. Following a peak at day 15-28 postinfection, the IgG antibody response and plasma neutralizing titers gradually decreased over time but stabilized after 6 months. Compared to G614, plasma neutralizing titers were more than 8-fold lower against variants Beta, Gamma, and Delta. SARS-CoV-2-specific memory B and T cells persisted in the majority of patients up to 15 months although a significant decrease in specific T cells, but not B cells, was observed between 6 and 15 months. Antiviral specific immunity, especially memory B cells in COVID-19 convalescent patients, is long-lasting, but some variants of concern may at least partially escape the neutralizing activity of plasma antibodies.
Keywords: Immune response; Immunology; Virology.
© 2022 The Author(s).
Conflict of interest statement
The other authors declare no competing interests.
Figures







References
-
- Addetia A., Crawford K.H.D., Dingens A., Zhu H., Roychoudhury P., Huang M.L., Jerome K.R., Bloom J.D., Greninger A.L. Neutralizing antibodies correlate with protection from SARS-CoV-2 in humans during a fishery vessel outbreak with a high attack rate. J. Clin. Microbiol. 2020;58:e02107–e02120. doi: 10.1128/JCM.02107-20. - DOI - PMC - PubMed
-
- Anand S.P., Prevost J., Nayrac M., Beaudoin-Bussieres G., Benlarbi M., Gasser R., Brassard N., Laumaea A., Gong S.Y., Bourassa C., et al. Longitudinal analysis of humoral immunity against SARS-CoV-2 spike in convalescent individuals up to 8 months post-symptom onset. Cell Rep. Med. 2021;2:100290. doi: 10.1016/j.xcrm.2021.100290. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

