This is a preprint.
Older Adults Mount Less Durable Humoral Responses to a Two-dose COVID-19 mRNA Vaccine Regimen, but Strong Initial Responses to a Third Dose
- PMID: 35018381
- PMCID: PMC8750654
- DOI: 10.1101/2022.01.06.22268745
Older Adults Mount Less Durable Humoral Responses to a Two-dose COVID-19 mRNA Vaccine Regimen, but Strong Initial Responses to a Third Dose
Update in
-
Older Adults Mount Less Durable Humoral Responses to Two Doses of COVID-19 mRNA Vaccine but Strong Initial Responses to a Third Dose.J Infect Dis. 2022 Sep 21;226(6):983-994. doi: 10.1093/infdis/jiac199. J Infect Dis. 2022. PMID: 35543278 Free PMC article.
Abstract
Background: Third COVID-19 vaccine doses are broadly recommended, but immunogenicity data remain limited, particularly in older adults.
Methods: We measured circulating antibodies against the SARS-CoV-2 spike protein receptor-binding domain, ACE2 displacement, and virus neutralization against ancestral and Omicron (BA.1) strains from pre-vaccine up to one month following the third dose, in 151 adults aged 24-98 years who received COVID-19 mRNA vaccines.
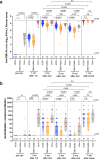
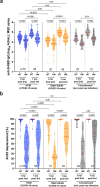
Results: Following two vaccine doses, humoral immunity was weaker, less functional and less durable in older adults, where a higher number of chronic health conditions was a key correlate of weaker responses and poorer durability. Third doses boosted antibody binding and function to higher levels than second-doses, and induced responses in older adults that were comparable in magnitude to those in younger adults. Humoral responses against Omicron were universally weaker than against the ancestral strain after both second and third doses; nevertheless, after three doses, anti-Omicron responses in older adults reached equivalence to those in younger adults. After three vaccine doses, the number of chronic health conditions, but not age per se, was the strongest consistent correlate of weaker humoral responses.
Conclusion: Results underscore the immune benefits of third COVID-19 vaccine doses, particularly in older adults.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
