Targeting RUNX1 as a novel treatment modality for pulmonary arterial hypertension
- PMID: 35018410
- PMCID: PMC9799056
- DOI: 10.1093/cvr/cvac001
Targeting RUNX1 as a novel treatment modality for pulmonary arterial hypertension
Abstract
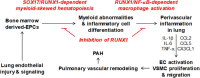
Aims: Pulmonary arterial hypertension (PAH) is a fatal disease without a cure. Previously, we found that transcription factor RUNX1-dependent haematopoietic transformation of endothelial progenitor cells may contribute to the pathogenesis of PAH. However, the therapeutic potential of RUNX1 inhibition to reverse established PAH remains unknown. In the current study, we aimed to determine whether RUNX1 inhibition was sufficient to reverse Sugen/hypoxia (SuHx)-induced pulmonary hypertension (PH) in rats. We also aimed to demonstrate possible mechanisms involved.
Methods and results: We administered a small molecule specific RUNX1 inhibitor Ro5-3335 before, during, and after the development of SuHx-PH in rats to investigate its therapeutic potential. We quantified lung macrophage recruitment and activation in vivo and in vitro in the presence or absence of the RUNX1 inhibitor. We generated conditional VE-cadherin-CreERT2; ZsGreen mice for labelling adult endothelium and lineage tracing in the SuHx-PH model. We also generated conditional Cdh5-CreERT2; Runx1(flox/flox) mice to delete Runx1 gene in adult endothelium and LysM-Cre; Runx1(flox/flox) mice to delete Runx1 gene in cells of myeloid lineage, and then subjected these mice to SuHx-PH induction. RUNX1 inhibition in vivo effectively prevented the development, blocked the progression, and reversed established SuHx-induced PH in rats. RUNX1 inhibition significantly dampened lung macrophage recruitment and activation. Furthermore, lineage tracing with the inducible VE-cadherin-CreERT2; ZsGreen mice demonstrated that a RUNX1-dependent endothelial to haematopoietic transformation occurred during the development of SuHx-PH. Finally, tissue-specific deletion of Runx1 gene either in adult endothelium or in cells of myeloid lineage prevented the mice from developing SuHx-PH, suggesting that RUNX1 is required for the development of PH.
Conclusion: By blocking RUNX1-dependent endothelial to haematopoietic transformation and pulmonary macrophage recruitment and activation, targeting RUNX1 may be as a novel treatment modality for pulmonary arterial hypertension.
Keywords: Endothelial cells; Macrophages; Pulmonary hypertension; RUNX1.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: none declared.
Figures






References
-
- Farber HW, Loscalzo J.. Pulmonary arterial hypertension. N Engl J Med 2004;351:1655–1665. - PubMed
-
- Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A.. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004;43:5S–12S. - PubMed
-
- Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar R, Landzberg M, Machado RF, Olschewski H, Robbins IM, Souza R.. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62:D34–D41. - PubMed
-
- McLaughlin VV, Shah SJ, Souza R, Humbert M.. Management of pulmonary arterial hypertension. J Am Coll Cardiol 2015;65:1976–1997. - PubMed
-
- Ventetuolo CE, Klinger JR.. WHO Group 1 pulmonary arterial hypertension: current and investigative therapies. Prog Cardiovasc Dis 2012;55:89–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

