Determinants of the temperature adaptation of mRNA degradation
- PMID: 35018460
- PMCID: PMC8789057
- DOI: 10.1093/nar/gkab1261
Determinants of the temperature adaptation of mRNA degradation
Abstract
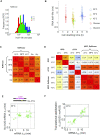
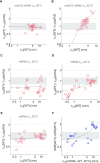
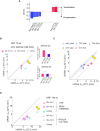
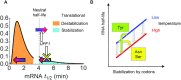
The rate of chemical reactions increases proportionally with temperature, but the interplay of biochemical reactions permits deviations from this relation and adaptation. The degradation of individual mRNAs in yeast increased to varying degrees with temperature. We examined how these variations are influenced by the translation and codon composition of mRNAs. We developed a method that revealed the existence of a neutral half-life above which mRNAs are stabilized by translation but below which they are destabilized. The proportion of these two mRNA subpopulations remained relatively constant under different conditions, even with slow cell growth due to nutrient limitation, but heat shock reduced the proportion of translationally stabilized mRNAs. At the same time, the degradation of these mRNAs was partially temperature-compensated through Upf1, the mediator of nonsense-mediated decay. Compensation was also promoted by some asparagine and serine codons, whereas tyrosine codons promote temperature sensitization. These codons play an important role in the degradation of mRNAs encoding key cell membrane and cell wall proteins, which promote cell integrity.
© The Author(s) 2022. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Alster C.J., Fischer J.C., Allison S.D., Treseder K.K.. Embracing a new paradigm for temperature sensitivity of soil microbes. Glob. Chang. Biol. 2020; 26:3221–3229. - PubMed
-
- Kortmann J., Narberhaus F.. Bacterial RNA thermometers: molecular zippers and switches. Nat. Rev. Microbiol. 2012; 10:255–265. - PubMed
-
- Tomita J., Nakajima M., Kondo T., Iwasaki H.. No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science. 2005; 307:251–254. - PubMed
-
- Robertson R.M., Money T.G.. Temperature and neuronal circuit function: compensation, tuning and tolerance. Curr. Opin. Neurobiol. 2012; 22:724–734. - PubMed
-
- Irvine S.Q. Embryonic canalization and its limits-A view from temperature. J. Exp. Zool. B Mol. Dev. Evol. 2020; 334:128–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

