Neonatal pulmonary hypertension after severe early-onset fetal growth restriction: post hoc reflections on the Dutch STRIDER study
- PMID: 35018508
- PMCID: PMC8964651
- DOI: 10.1007/s00431-021-04355-x
Neonatal pulmonary hypertension after severe early-onset fetal growth restriction: post hoc reflections on the Dutch STRIDER study
Abstract
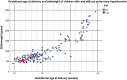
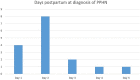
The aim was to reflect on the unexpected finding of persistent pulmonary hypertension of the neonate (PPHN) and pulmonary hypertension in infants born within the Dutch STRIDER trial, its definition and possible pathophysiological mechanisms. The trial randomly assigned pregnant women with severe early-onset fetal growth restriction to sildenafil 25 mg three times a day versus placebo. Sildenafil use did not reduce perinatal mortality and morbidity, but did result in a higher rate of neonatal pulmonary hypertension (PH). The current paper reflects on the used definition, prevalence, and possible pathophysiology of the data on pulmonary hypertension. Twenty infants were diagnosed with pulmonary hypertension (12% of 163 live born infants). Of these, 16 infants had PPHN shortly after birth, and four had pulmonary hypertension associated with sepsis or bronchopulmonary dysplasia. Four infants with PPHN in the early neonatal period subsequently developed pulmonary hypertension associated with bronchopulmonary dysplasia in later life. Infants with pulmonary hypertension were at lower gestational age at delivery, had a lower birth weight and a higher rate of neonatal co-morbidity. The infants in the sildenafil group showed a significant increase in pulmonary hypertension compared to the placebo group (relative risk 3.67; 95% confidence interval 1.28 to 10.51, P = 0.02).
Conclusion: Pulmonary hypertension occurred more frequent among infants of mothers allocated to antenatal sildenafil compared with placebo. A possible pathophysiological mechanism could be a "rebound" vasoconstriction after cessation of sildenafil. Additional studies and data are necessary to understand the mechanism of action.
What is known: • In the Dutch STRIDER trial, persistent pulmonary hypertension in the neonate (PPHN) was more frequent among infants after antenatal sildenafil exposure versus placebo.
What is new: • The current analysis focuses on the distinction between PPHN and pulmonary hypertension associated with sepsis or bronchopulmonary dysplasia and on timing of diagnosis and aims to identify the infants at risk for developing pulmonary hypertension. • The diagnosis pulmonary hypertension is complex, especially in infants born after severe early-onset fetal growth restriction. The research field could benefit from an unambiguous consensus definition and standardized screening in infants at risk is proposed.
Keywords: Fetal growth restriction; Neonatal morbidity; Neonatal mortality; Pulmonary hypertension; Sildenafil.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Comment in
-
The antenatal sildenafil STRIDER trial for severe fetal growth restriction, are post hoc reflections ad rem?Eur J Pediatr. 2022 Oct;181(10):3775-3776. doi: 10.1007/s00431-022-04569-7. Epub 2022 Jul 26. Eur J Pediatr. 2022. PMID: 35879469 No abstract available.
Similar articles
-
Maternal Sildenafil vs Placebo in Pregnant Women With Severe Early-Onset Fetal Growth Restriction: A Randomized Clinical Trial.JAMA Netw Open. 2020 Jun 1;3(6):e205323. doi: 10.1001/jamanetworkopen.2020.5323. JAMA Netw Open. 2020. PMID: 32585017 Free PMC article. Clinical Trial.
-
Detailed statistical analysis plan for the Dutch STRIDER (Sildenafil TheRapy In Dismal prognosis Early-onset fetal growth Restriction) randomised clinical trial on sildenafil versus placebo for pregnant women with severe early onset fetal growth restriction.Trials. 2019 Jan 11;20(1):42. doi: 10.1186/s13063-018-3136-z. Trials. 2019. PMID: 30635020 Free PMC article.
-
STRIDER (Sildenafil TheRapy in dismal prognosis early onset fetal growth restriction): an international consortium of randomised placebo-controlled trials.BMC Pregnancy Childbirth. 2017 Dec 28;17(1):440. doi: 10.1186/s12884-017-1594-z. BMC Pregnancy Childbirth. 2017. PMID: 29282009 Free PMC article. Clinical Trial.
-
The efficacy of sildenafil therapy in dismal prognosis early-onset intrauterine growth restriction: the STRIDER RCT.Southampton (UK): National Institute for Health and Care Research; 2024 Oct. Southampton (UK): National Institute for Health and Care Research; 2024 Oct. PMID: 39591485 Free Books & Documents. Review.
-
Effect of L-arginine and sildenafil citrate on intrauterine growth restriction fetuses: a meta-analysis.BMC Pregnancy Childbirth. 2016 Aug 16;16:225. doi: 10.1186/s12884-016-1009-6. BMC Pregnancy Childbirth. 2016. PMID: 27528012 Free PMC article. Review.
Cited by
-
Placental syndromes and maternal cardiovascular health.Clin Sci (Lond). 2023 Aug 31;137(16):1211-1224. doi: 10.1042/CS20211130. Clin Sci (Lond). 2023. PMID: 37606085 Free PMC article. Review.
-
Phosphodiesterase Inhibitors in Fetal Growth Restriction: Do Not Forget to Consider Fetal Sex and Subcellular Compartmentation.Biomedicines. 2024 Oct 14;12(10):2329. doi: 10.3390/biomedicines12102329. Biomedicines. 2024. PMID: 39457641 Free PMC article.
-
Fetal sex and the relative reactivity of human umbilical vein and arteries are key determinants in potential beneficial effects of phosphodiesterase inhibitors.J Appl Physiol (1985). 2024 Jun 1;136(6):1526-1545. doi: 10.1152/japplphysiol.00540.2023. Epub 2024 May 2. J Appl Physiol (1985). 2024. PMID: 38695358 Free PMC article.
-
OLA1 Phosphorylation Governs the Mitochondrial Bioenergetic Function of Pulmonary Vascular Cells.Am J Respir Cell Mol Biol. 2023 Apr;68(4):395-405. doi: 10.1165/rcmb.2022-0186OC. Am J Respir Cell Mol Biol. 2023. PMID: 36481055 Free PMC article.
-
Intravenous sildenafil for treatment of early pulmonary hypertension in preterm infants.Sci Rep. 2023 May 24;13(1):8405. doi: 10.1038/s41598-023-35387-y. Sci Rep. 2023. PMID: 37225769 Free PMC article.
References
-
- Pels A, Derks J, Elvan-Taspinar A, van Drongelen J, de Boer M, Duvekot H, van Laar J, van Eyck J, Al-Nasiry S, Sueters M, Post M (2020) Maternal sildenafil vs placebo in pregnant women with severe early-onset fetal growth restriction: a randomized clinical trial. JAMA Netw Open 3(6):e205323 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical