Alkbh1-mediated DNA N6-methyladenine modification regulates bone marrow mesenchymal stem cell fate during skeletal aging
- PMID: 35018683
- PMCID: PMC8828262
- DOI: 10.1111/cpr.13178
Alkbh1-mediated DNA N6-methyladenine modification regulates bone marrow mesenchymal stem cell fate during skeletal aging
Abstract
Objectives: DNA N6-methyladenine (N6-mA) demethylase Alkbh1 participates in regulating osteogenic differentiation of mesenchymal stem cell (MSCs) and vascular calcification. However, the role of Alkbh1 in bone metabolism remains unclear.
Materials and methods: Bone marrow mesenchymal stem cells (BMSCs)-specific Alkbh1 knockout mice were used to investigate the role of Alkbh1 in bone metabolism. Western blot, qRT-PCR, and immunofluorescent staining were used to evaluate the expression of Alkbh1 or optineurin (optn). Micro-CT, histomorphometric analysis, and calcein double-labeling assay were used to evaluate bone phenotypes. Cell staining and qRT-PCR were used to evaluate the osteogenic or adipogenic differentiation of BMSCs. Dot blotting was used to detect the level of N6-mA in genomic DNA. Chromatin immunoprecipitation (Chip) assays were used to identify critical targets of Alkbh1. Alkbh1 adeno-associated virus was used to overexpress Alkbh1 in aged mice.
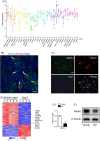
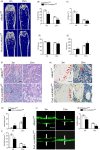
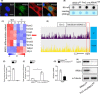
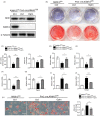
Results: Alkbh1 expression in BMSCs declined during aging. Knockout of Alkbh1 promoted adipogenic differentiation of BMSCs while inhibited osteogenic differentiation. BMSC-specific Alkbh1 knockout mice exhibited reduced bone mass and increased marrow adiposity. Mechanistically, we identified optn as the downstream target through which Alkbh1-mediated DNA m6A modification regulated BMSCs fate. Overexpression of Alkbh1 attenuated bone loss and marrow fat accumulation in aged mice.
Conclusions: Our findings demonstrated that Alkbh1 regulated BMSCs fate and bone-fat balance during skeletal aging and provided a potential target for the treatment of osteoporosis.
Keywords: Alkbh1; BMSCs; aging; epigenetic; osteoporosis.
© 2022 The Authors. Cell Proliferation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures


References
-
- Duque G, Rivas D, Li W, et al. Age‐related bone loss in the LOU/c rat model of healthy ageing. Exp Gerontol. 2009;44(3):183‐189. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

