Membrane vesicles from antibiotic-resistant Staphylococcus aureus transfer antibiotic-resistance to antibiotic-susceptible Escherichia coli
- PMID: 35019198
- PMCID: PMC9306644
- DOI: 10.1111/jam.15449
Membrane vesicles from antibiotic-resistant Staphylococcus aureus transfer antibiotic-resistance to antibiotic-susceptible Escherichia coli
Abstract
Aim: Bacteria naturally produce membrane vesicles (MVs), which have been shown to contribute to the spread of multi-drug resistant bacteria (MDR) by delivering antibiotic-resistant substances to antibiotic-susceptible bacteria. Here, we aim to show that MVs from Gram-positive bacteria are capable of transferring β-lactam antibiotic-resistant substances to antibiotic-sensitive Gram-negative bacteria.
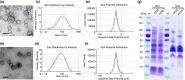
Materials and methods: MVs were collected from a methicillin-resistant strain of Staphylococcus aureus (MRSA) and vesicle-mediated fusion with antimicrobial-sensitive Escherichia coli (RC85). It was performed by exposing the bacteria to the MVs to develop antimicrobial-resistant E. coli (RC85-T).
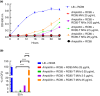
Results: The RC85-T exhibited a higher resistance to β-lactam antibiotics compared to the parent strain. Although the secretion rates of the MVs from RC85-T and the parent strain were nearly equal, the β-lactamase activity of the MVs from RC85-T was 12-times higher than that of MVs from the parent strain, based on equivalent protein concentrations. Moreover, MVs secreted by RC85-T were able to protect β-lactam-susceptible E. coli from β-lactam antibiotic-induced growth inhibition in a dose-dependent manner.
Conclusion: MVs play a role in transferring substances from Gram-positive to Gram-negative bacteria, shown by the release of MVs from RC85-T that were able to protect β-lactam-susceptible bacteria from β-lactam antibiotics.
Significance and impact of study: MVs are involved in the emergence of antibiotic-resistant strains in a mixed bacterial culture, helping us to understand how the spread of multidrug-resistant bacteria could be reduced.
Keywords: antibiotic-resistant bacteria; antibiotic-susceptible bacteria; gram-negative bacteria; gram-positive bacteria; membrane vesicles (MVs); vesicle-mediated transferring of antimicrobial resistance.
© 2022 The Authors. Journal of Applied Microbiology published by John Wiley & Sons Ltd on behalf of Society for Applied Microbiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Andreoni, F. , Toyofuku, M. , Menzi, C. , Kalawong, R. , Mairpady, S.S. , François, P. et al. (2019) Antibiotics stimulate formation of vesicles in Staphylococcus aureus in both phage‐dependent and‐independent fashions and via different routes. Antimicrobial Agents and Chemotherapy, 63, e01439–18. - PMC - PubMed
-
- Andrews, J.M. (2001) Determination of minimum inhibitory concentrations. The Journal of Antimicrobial Chemotherapy, 48, 5–16. - PubMed
-
- Centers for Disease Control and Prevention . (2013) Antibiotic resistance threats in the United States, 2013. Atlanta, GA: CDC. Available online: https://www.cdc.gov/drugresistance/threat‐report‐2013/pdf/arthreats‐2013.... .
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

