Highly Efficient Polarizing Agents for MAS-DNP of Proton-Dense Molecular Solids
- PMID: 35019217
- PMCID: PMC8901535
- DOI: 10.1002/anie.202114103
Highly Efficient Polarizing Agents for MAS-DNP of Proton-Dense Molecular Solids
Abstract
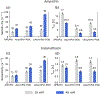
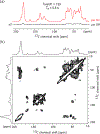
Efficiently hyperpolarizing proton-dense molecular solids through dynamic nuclear polarization (DNP) solid-state NMR is still an unmet challenge. Polarizing agents (PAs) developed so far do not perform well on proton-rich systems, such as organic microcrystals and biomolecular assemblies. Herein we introduce a new PA, cAsymPol-POK, and report outstanding hyperpolarization efficiency on 12.76 kDa U-13 C,15 N-labeled LecA protein and pharmaceutical drugs at high magnetic fields (up to 18.8 T) and fast magic angle spinning (MAS) frequencies (up to 40 kHz). The performance of cAsymPol-POK is rationalized by MAS-DNP simulations combined with electron paramagnetic resonance (EPR), density functional theory (DFT) and molecular dynamics (MD). This work shows that this new biradical is compatible with challenging biomolecular applications and unlocks the rapid acquisition of 13 C-13 C and 15 N-13 C correlations of pharmaceutical drugs at natural isotopic abundance, which are key experiments for structure determination.
Keywords: Biomolecules; Dynamic Nuclear Polarization; MAS-DNP; Nuclear Magnetic Resonance; Pharmaceuticals; Polarizing Agents.
© 2022 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest
Figures




References
-
- Overhauser AW, Phys. Rev. 1953, 92, 411–415.
-
- Lilly Thankamony AS, Wittmann JJ, Kaushik M, Corzilius B, Prog. Nucl. Magn. Reson. Spectrosc. 2017, 102, 120–195. - PubMed
-
- Lee D, Hediger S, De Paëpe G, Solid State Nucl. Magn. Reson. 2015, 66, 6–20. - PubMed
-
- Plainchont B, Berruyer P, Dumez J-N, Jannin S, Giraudeau P, Anal. Chem. 2018, 90, 3639–3650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

