Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia
- PMID: 35019853
- PMCID: PMC9924295
- DOI: 10.1158/2643-3230.BCD-21-0095
Next-Generation Sequencing of Minimal Residual Disease for Predicting Relapse after Tisagenlecleucel in Children and Young Adults with Acute Lymphoblastic Leukemia
Abstract
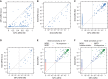
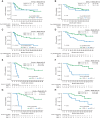
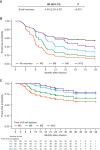
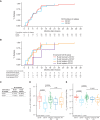
We assessed minimal residual disease (MRD) detection and B-cell aplasia after tisagenlecleucel therapy for acute lymphoblastic leukemia (ALL) to define biomarkers predictive of relapse (N = 143). Next-generation sequencing (NGS) MRD detection >0 in bone marrow (BM) was highly associated with relapse. B-cell recovery [signifying loss of functional chimeric antigen receptor (CAR) T cells] within the first year of treatment was associated with a hazard ratio (HR) for relapse of 4.5 [95% confidence interval (CI), 2.03-9.97; P < 0.001]. Multivariate analysis at day 28 showed independent associations of BMNGS-MRD >0 (HR = 4.87; 95% CI, 2.18-10.8; P < 0.001) and B-cell recovery (HR = 3.33; 95% CI, 1.44-7.69; P = 0.005) with relapse. By 3 months, the BMNGS-MRD HR increased to 12 (95% CI, 2.87-50; P < 0.001), whereas B-cell recovery was not independently predictive (HR = 1.27; 95% CI, 0.33-4.79; P = 0.7). Relapses occurring with persistence of B-cell aplasia were largely CD19- (23/25: 88%). Detectable BMNGS-MRD reliably predicts risk with sufficient time to consider approaches to relapse prevention such as hematopoietic cell transplantation (HCT) or second CAR-T cell infusion. SIGNIFICANCE: Detectable disease by BMNGS-MRD with or without B-cell aplasia is highly predictive of relapse after tisagenlecleucel therapy for ALL. Clonotypic rearrangements used to follow NGS-MRD did not change after loss of CD19 or lineage switch. High-risk patients identified by these biomarkers may benefit from HCT or investigational cell therapies.See related commentary by Ghorashian and Bartram, p. 2.This article is highlighted in the In This Issue feature, p. 1.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures


Comment in
-
Rare Sequences Make Sense of CAR T-cell Therapy Outcomes.Blood Cancer Discov. 2022 Jan;3(1):2-4. doi: 10.1158/2643-3230.BCD-21-0199. Epub 2021 Dec 1. Blood Cancer Discov. 2022. PMID: 35019857 Free PMC article.
References
-
- Emily Whitehead Foundation [homepage on the Internet]. Philipsburg (PA): Emily Whitehead Foundation; 2014–2021 [cited 2021 Oct 28]. Available from: https://emilywhiteheadfoundation.org/our-journey/.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

