SARS-CoV-2 BNT162b2 vaccine-induced humoral response and reactogenicity in individuals with prior COVID-19 disease
- PMID: 35019861
- PMCID: PMC8876462
- DOI: 10.1172/jci.insight.155889
SARS-CoV-2 BNT162b2 vaccine-induced humoral response and reactogenicity in individuals with prior COVID-19 disease
Abstract
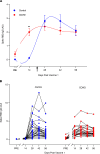
BACKGROUNDMost individuals with prior COVID-19 disease manifest long-term protective immune responses against reinfection. Accordingly, we tested the hypothesis that humoral immune and reactogenicity responses to a SARS-CoV-2 mRNA vaccine differ in individuals with and without prior COVID-19 disease.METHODSHealth care workers (n = 61) with (n = 30) and without (n = 31) prior COVID-19 disease received two 30 μg doses of Pfizer BNT162b2 vaccine 3 weeks apart. Serum IgG antibody against the spike receptor-binding domain; serum neutralizing activity; and vaccine reactogenicity were assessed longitudinally every 2 weeks for 56 days after the first injection.RESULTSThe COVID-19 group manifested more rapid increases in spike IgG antibody and serum neutralizing activity after the first vaccine dose but showed little or no increase after the second dose compared with the infection-naive group. In fact, spike IgG was at its maximum level after the first dose in 36% of the COVID-19 group versus 0% of the infection-naive group. Peak IgG antibody levels were lower but appeared to fall more slowly in the COVID-19 group versus the infection-naive group. Finally, adverse systemic reactions, e.g., fever, headache, and malaise, were more frequent and lasted longer after both the first and second injection in the COVID-19 group than in the infection-naive group.CONCLUSIONIndividuals with prior COVID-19 disease demonstrate a robust, accelerated humoral immune response to the first dose but an attenuated response to the second dose of BNT162b2 vaccine compared with controls. The COVID-19 group also experienced greater reactogenicity. Humoral responses and reactogenicity to BNT162b2 differ qualitatively and quantitatively in individuals with prior COVID-19 disease compared with infection-naive individuals.FUNDINGThis work was supported by Temple University institutional funds.
Keywords: Adaptive immunity; COVID-19.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

