Cyclopentannulated Dihydrotetraazapentacenes
- PMID: 35020239
- PMCID: PMC9303544
- DOI: 10.1002/chem.202104203
Cyclopentannulated Dihydrotetraazapentacenes
Abstract
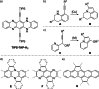
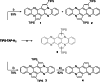
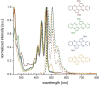
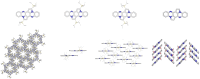
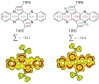
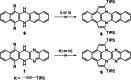
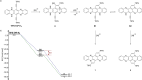
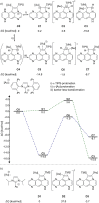
The transition-metal-catalyzed cyclization of bissilylethynylated N,N'-dihydrotetraazapentacene (TIPS-TAP-H2 ) into bissilylated cyclopenta[fg,qr]pentacenes is reported. Depending on the catalyst either none, one or two silyl groups migrate and change their positions in the formed five-membered rings. The optoelectronic properties are quite similar, whereas the packing motifs differ dramatically. Control experiments and quantum chemical calculations were performed to investigate the mechanism of the reaction and the selectivity of the silyl shift.
Keywords: aromaticity; azaarenes; catalysis; cycloisomerization; cyclopentannulation; silyl shift.
© 2022 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Miao S., Appleton A. L., Berger N., Barlow S., Marder S. R., Hardcastle K. I., Bunz U. H. F., Chem. Eur. J. 2009, 15, 4990–4993. - PubMed
-
- For an selected overview of metal-catalyzed cycloisomerization reactions of heteroatom-functionalized alkynes:
-
- Hendrich C. M., Sekine K., Koshikawa T., Tanaka K., Hashmi A. S. K., Chem. Rev. 2021, 121, 9113–9163; - PubMed
-
- Praveen C., Chem. Rec. 2021, 21, 1697–1737; - PubMed
-
- Chung L.-H., Yeung C.-F., Wong C.-Y., Chem. Eur. J. 2020, 26,6102–6112; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

