"Fix and Click" for Assay of Sphingolipid Signaling in Single Primary Human Intestinal Epithelial Cells
- PMID: 35020354
- PMCID: PMC8931668
- DOI: 10.1021/acs.analchem.1c03503
"Fix and Click" for Assay of Sphingolipid Signaling in Single Primary Human Intestinal Epithelial Cells
Abstract
Capillary electrophoresis with fluorescence detection (CE-F) is a powerful method to measure enzyme activation in single cells. However, cellular enzymatic assays used in CE-F routinely utilize reporter substrates that possess a bulky fluorophore that may impact enzyme kinetics. To address these challenges, we describe a "fix and click" method utilizing an alkyne-terminated enzyme activation reporter, aldehyde-based fixation, and a click chemistry reaction to attach a fluorophore prior to analysis by single-cell CE-F. The "fix and click" strategy was utilized to investigate sphingolipid signaling in both immortalized cell lines and primary human colonic epithelial cells. When the sphingosine alkyne reporter was loaded into cells, this reporter was metabolized to ceramide (31.6 ± 3.3% peak area) without the production of sphingosine-1-phosphate. In contrast, when the reporter sphingosine fluorescein was introduced into cells, sphingosine fluorescein was converted to sphingosine-1-phosphate and downstream products (32.8 ± 5.7% peak area) without the formation of ceramide. Sphingolipid metabolism was measured in single cells from both differentiated and stem/proliferative human colonic epithelium using "fix and click" paired with CE-F to highlight the diversity of sphingosine metabolism in single cells from primary human colonic epithelium. This novel method will find widespread utility for the performance of single-cell enzyme assays by virtue of its ability to temporally and spatially separate cellular reactions with alkyne-terminated reporters, followed by the assay of enzyme activation at a later time and place.
Conflict of interest statement
Notes
Y.W. and N.L.A. disclose a financial interest in Altis Biosystems, Inc. L.A.G., A.M., M.Y., B.V.P., Q.Z., W.H., A.J.C., and Q.Z. declare no financial conflict.
Figures



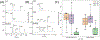
References
-
- Evangelisti C; Evangelisti C; Buontempo F; Lonetti A; Orsini E; Chiarini F; Barata JT; Pyne S; Pyne NJ; Martelli AM Therapeutic Potential of Targeting Sphingosine Kinases and Sphingosine 1-Phosphate in Hematological Malignancies. Leukemia 2016, 30 (11), 2142–2151. DOI: 10.1038/leu.2016.208. - DOI - PubMed
-
- Paugh SW; Paugh BS; Rahmani M; Kapitonov D; Almenara JA; Kordula T; Milstien S; Adams JK; Zipkin RE; Grant S; Spiegel S A Selective Sphingosine Kinase 1 Inhibitor Integrates Multiple Molecular Therapeutic Targets in Human Leukemia. Blood 2008, 112 (4), 1382–1391. 10.1182/blood-2008-02-138958. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

