A Pilot Study for the Evaluation of an Interferon Gamma Release Assay (IGRA) To Measure T-Cell Immune Responses after SARS-CoV-2 Infection or Vaccination in a Unique Cloistered Cohort
- PMID: 35020419
- PMCID: PMC8925901
- DOI: 10.1128/jcm.02199-21
A Pilot Study for the Evaluation of an Interferon Gamma Release Assay (IGRA) To Measure T-Cell Immune Responses after SARS-CoV-2 Infection or Vaccination in a Unique Cloistered Cohort
Abstract
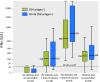
Assessment of T-cell responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antigens may be of value to determine long-lasting protection to breakthrough infections or reinfections. Interferon gamma release assay is a validated method to test cellular immunity in mycobacterial infections and has been proposed for patients with SARS-CoV-2 infection or vaccination. Quantitative IgG to spike and qualitative IgG to nucleocapsid antigens were determined by chemiluminescence microparticle immunoassay using the Architect platform (Abbott), and interferon gamma release assays against two Qiagen proprietary mixes of SARS-CoV-2 spike protein (antigen 1 and antigen 2) were performed for a selected group of subjects. A total of 121 subjects in a cloistered institution after a COVID-19 outbreak was studied. IgG spike levels and interferon gamma concentrations were highest among subjects after two doses of vaccine, followed by patients with a longer history of past COVID-19 and no vaccination. The best cutoff for the interferon gamma assay was 25 IU/L for all subgroups of individuals and the two sets of SARS-CoV-2 antigens studied. Testing T-cell response may be of clinical utility to determine immunity after exposure to SARS-CoV-2 antigens, with the interferon gamma concentration of 25 IU/L as the best cutoff either after infection or vaccination.
Keywords: COVID-19; IGRA; SARS-CoV-2; interferon gamma release assay; serology; vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
We declare no conflict of interest.
Figures
References
-
- Schäfer A, Muecksch F, Lorenzi JCC, Leist SR, Cipolla M, Bournazos S, Schmidt F, Maison RM, Gazumyan A, Martinez DR, Baric RS, Robbiani DF, Hatziioannou T, Ravetch JV, Bieniasz PD, Bowen RA, Nussenzweig MC, Sheahan TP. 2021. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J Exp Med 218:e20201993. 10.1084/jem.20201993. - DOI - PMC - PubMed
-
- Garcia-Beltran WF, Lam EC, Astudillo MG, Yang D, Miller TE, Feldman J, Hauser BM, Caradonna TM, Clayton KL, Nitido AD, Murali MR, Alter G, Charles RC, Dighe A, Branda JA, Lennerz JK, Lingwood D, Schmidt AG, Iafrate AJ, Balazs AB. 2021. COVID-19-neutralizing antibodies predict disease severity and survival. Cell 184:476–488. 10.1016/j.cell.2020.12.015. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous