Imaging and tracing the pattern of adult ovarian angiogenesis implies a strategy against female reproductive aging
- PMID: 35020427
- PMCID: PMC8754302
- DOI: 10.1126/sciadv.abi8683
Imaging and tracing the pattern of adult ovarian angiogenesis implies a strategy against female reproductive aging
Abstract
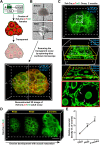
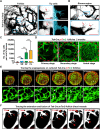
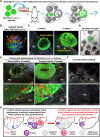
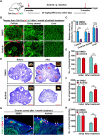
Robust angiogenesis is continuously active in ovaries to remodel the ovary-body connections in mammals, but understanding of this unique process remains elusive. Here, we performed high-resolution, three-dimensional ovarian vascular imaging and traced the pattern of ovarian angiogenesis and vascular development in the long term. We found that angiogenesis was mainly active on ovarian follicles and corpus luteum and that robust angiogenesis constructs independent but temporary vascular networks for each follicle. Based on the pattern of ovarian angiogenesis, we designed an angiogenesis-blocking strategy by axitinib administration to young females, and we found that the temporary suppression of angiogenesis paused ovarian development and kept the ovarian reserve in the long term, leading to postponed ovarian senescence and an extension of the female reproductive life span. Together, by uncovering the detailed model of physiological ovarian angiogenesis, our experiments suggest a potential approach to delay female reproductive aging through the manipulation of angiogenesis.
Figures

References
-
- Carmeliet P., Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005). - PubMed
-
- Cao Y. H., Adipose tissue angiogenesis as a therapeutic target for obesity and metabolic diseases. Nat. Rev. Drug Discov. 9, 107–115 (2010). - PubMed
-
- Cao Y., Angiogenesis and vascular functions in modulation of obesity, adipose metabolism, and insulin sensitivity. Cell Metab. 18, 478–489 (2013). - PubMed
-
- Robinson R. S., The critical importance of ovarian angiogenesis. Reprod. Fertil. Dev. 25, iii–v (2013). - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

