PP2A-dependent TFEB activation is blocked by PIKfyve-induced mTORC1 activity
- PMID: 35020443
- PMCID: PMC9250387
- DOI: 10.1091/mbc.E21-06-0309
PP2A-dependent TFEB activation is blocked by PIKfyve-induced mTORC1 activity
Abstract
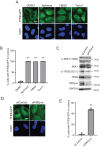
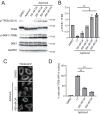
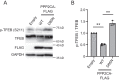
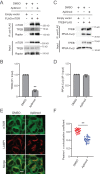
Transcriptional factor EB (TFEB) is a master regulator of genes required for autophagy and lysosomal function. The nuclear localization of TFEB is blocked by the mechanistic target of rapamycin complex 1 (mTORC1)-dependent phosphorylation of TFEB at multiple sites including Ser-211. Here we show that inhibition of PIKfyve, which produces phosphatidylinositol 3,5-bisphosphate on endosomes and lysosomes, causes a loss of Ser-211 phosphorylation and concomitant nuclear localization of TFEB. We found that while mTORC1 activity toward S6K1, as well as other major mTORC1 substrates, is not impaired, PIKfyve inhibition specifically impedes the interaction of TFEB with mTORC1. This suggests that mTORC1 activity on TFEB is selectively inhibited due to loss of mTORC1 access to TFEB. In addition, we found that TFEB activation during inhibition of PIKfyve relies on the ability of protein phosphatase 2A (PP2A) but not calcineurin/PPP3 to dephosphorylate TFEB Ser-211. Thus when PIKfyve is inhibited, PP2A is dominant over mTORC1 for control of TFEB phosphorylation at Ser-S211. Together these findings suggest that mTORC1 and PP2A have opposing roles on TFEB via phosphorylation and dephosphorylation of Ser-211, respectively, and further that PIKfyve inhibits TFEB activity by facilitating mTORC1-dependent phosphorylation of TFEB.
Figures



Similar articles
-
PIKFYVE-dependent regulation of MTORC1 and TFEB.Autophagy Rep. 2022 Jun 1;1(1):247-251. doi: 10.1080/27694127.2022.2082201. eCollection 2022. Autophagy Rep. 2022. PMID: 40396039 Free PMC article.
-
Lysosomotropic drugs activate TFEB via lysosomal membrane fluidization and consequent inhibition of mTORC1 activity.Cell Death Dis. 2018 Dec 13;9(12):1191. doi: 10.1038/s41419-018-1227-0. Cell Death Dis. 2018. PMID: 30546014 Free PMC article.
-
Multistep regulation of TFEB by MTORC1.Autophagy. 2017 Mar 4;13(3):464-472. doi: 10.1080/15548627.2016.1271514. Epub 2017 Jan 5. Autophagy. 2017. PMID: 28055300 Free PMC article.
-
TFEB: a double-edged sword for tumor metastasis.J Mol Med (Berl). 2023 Aug;101(8):917-929. doi: 10.1007/s00109-023-02337-0. Epub 2023 Jun 17. J Mol Med (Berl). 2023. PMID: 37328669 Review.
-
Past, present, and future perspectives of transcription factor EB (TFEB): mechanisms of regulation and association with disease.Cell Death Differ. 2022 Aug;29(8):1433-1449. doi: 10.1038/s41418-022-01028-6. Epub 2022 Jun 23. Cell Death Differ. 2022. PMID: 35739255 Free PMC article. Review.
Cited by
-
Selective Termination of Autophagy-Dependent Cancers.Cells. 2024 Jun 25;13(13):1096. doi: 10.3390/cells13131096. Cells. 2024. PMID: 38994949 Free PMC article. Review.
-
An AMBRA1, ULK1 and PP2A regulatory network regulates cytotoxic T cell differentiation via TFEB activation.Sci Rep. 2024 Dec 30;14(1):31838. doi: 10.1038/s41598-024-82957-9. Sci Rep. 2024. PMID: 39738384 Free PMC article.
-
mTORC1 signaling and diabetic kidney disease.Diabetol Int. 2024 Jun 20;15(4):707-718. doi: 10.1007/s13340-024-00738-1. eCollection 2024 Oct. Diabetol Int. 2024. PMID: 39469564 Free PMC article. Review.
-
Predictive, preventive, and personalized medicine in breast cancer: targeting the PI3K pathway.J Transl Med. 2024 Jan 3;22(1):15. doi: 10.1186/s12967-023-04841-w. J Transl Med. 2024. PMID: 38172946 Free PMC article. Review.
-
Roles of PIKfyve in multiple cellular pathways.Curr Opin Cell Biol. 2022 Jun;76:102086. doi: 10.1016/j.ceb.2022.102086. Epub 2022 May 16. Curr Opin Cell Biol. 2022. PMID: 35584589 Free PMC article. Review.
References
-
- Bago R, Malik N, Munson MJ, Prescott AR, Davies P, Sommer E, Shpiro N, Ward R, Cross D, Ganley IG, et al. (2014). Characterization of VPS34-IN1, a selective inhibitor of Vps34, reveals that the phosphatidylinositol 3-phosphate-binding SGK3 protein kinase is a downstream target of class III phosphoinositide 3-kinase. Biochem J 463, 413–427. - PMC - PubMed
-
- Cai X, Xu Y, Cheung AK, Tomlinson RC, Alcázar-Román A, Murphy L, Billich A, Zhang B, Feng Y, Klumpp M, et al. (2013). PIKfyve, a class III PI Kinase, is the target of the small molecular IL-12/IL-23 inhibitor apilimod and a player in toll-like receptor signaling. Chem Biol 20, 912–921. - PMC - PubMed
-
- Cai X, Xu Y, Kim Y-M, Loureiro J, Huang Q (2014). PIKfyve, a class III lipid kinase, is required for TLR-induced type I IFN production via modulation of ATF3. J Immunol 192, 3383–3389. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

