Efficacy and Safety of Dextroamphetamine Transdermal System for the Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents: Results from a Pivotal Phase 2 Study
- PMID: 35020462
- PMCID: PMC8972004
- DOI: 10.1089/cap.2021.0107
Efficacy and Safety of Dextroamphetamine Transdermal System for the Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents: Results from a Pivotal Phase 2 Study
Abstract
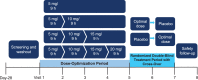
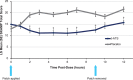
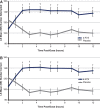
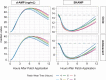
Objectives: To assess efficacy and safety of the new Dextroamphetamine Transdermal System (d-ATS) to treat children and adolescents (aged 6-17 years) with attention-deficit/hyperactivity disorder (ADHD). Methods: In this phase 2, randomized, placebo-controlled study, 4 d-ATS patches of differing doses (5, 10, 15, and 20 mg) were evaluated. Patients began a 5-week, open-label, stepwise dose-optimization period in which they received a 5-mg d-ATS patch (applied to hip) for 9 hours. During weekly visits, patients were evaluated for possible adjustments to the next dose level based on efficacy and safety. Once at the optimal dose, that dose was maintained during a 2-week, crossover double-blind treatment period. Primary endpoint was to assess efficacy of d-ATS versus placebo as measured by Swanson, Kotkin, Agler, M-Flynn, and Pelham Scale (SKAMP) total score; key secondary endpoints included assessing onset and duration of efficacy by SKAMP total score, and additional secondary endpoints included Permanent Product Measure of Performance (PERMP) scores. Safety was assessed throughout. Results: d-ATS treatment resulted in significant improvements versus placebo in ADHD symptoms as measured by SKAMP total score, with overall least-squares mean difference (95% confidence interval) versus placebo of -5.87 (6.76, -4.97; p < 0.001) over the 12-hour assessment period. Onset of efficacy was observed at 2 hours postdose (p < 0.001), and duration of effect continued through 12 hours (patch removed at 9 hours), with significant differences between d-ATS and placebo at all time points from 2 hours onward (all p ≤ 0.003). Significant improvements versus placebo in PERMP-A and PERMP-C scores were also observed from 2 to 12 hours postdose with d-ATS treatment. d-ATS was safe and well-tolerated, with a systemic safety profile similar to that observed with oral amphetamines. Conclusions: This study demonstrates that d-ATS is an effective and well-tolerated treatment for children and adolescents with ADHD. These data indicate that d-ATS can deliver sustained levels of efficacy along with the advantages of transdermal drug delivery, making it a beneficial new treatment option. Clinical Trial Registration no.: NCT01711021.
Keywords: ADHD; clinical trial; dextroamphetamine; transdermal.
Figures
Similar articles
-
d-Amphetamine Transdermal System in Treatment of Children and Adolescents with Attention-Deficit/Hyperactivity Disorder: Secondary Endpoint Results and Post Hoc Effect Size Analyses from a Pivotal Trial.J Child Adolesc Psychopharmacol. 2023 Jun;33(5):176-182. doi: 10.1089/cap.2023.0005. J Child Adolesc Psychopharmacol. 2023. PMID: 37339441 Free PMC article. Clinical Trial.
-
Early-Onset Efficacy and Safety Pilot Study of Amphetamine Extended-Release Oral Suspension in the Treatment of Children with Attention-Deficit/Hyperactivity Disorder.J Child Adolesc Psychopharmacol. 2019 Feb;29(1):2-8. doi: 10.1089/cap.2018.0078. Epub 2018 Dec 21. J Child Adolesc Psychopharmacol. 2019. PMID: 30575407 Free PMC article. Clinical Trial.
-
Efficacy and Safety of a Chewable Methylphenidate Extended-Release Tablet in Children with Attention-Deficit/Hyperactivity Disorder.J Child Adolesc Psychopharmacol. 2017 Oct;27(8):690-699. doi: 10.1089/cap.2016.0177. Epub 2017 May 30. J Child Adolesc Psychopharmacol. 2017. PMID: 28557548 Free PMC article. Clinical Trial.
-
The efficacy and safety profile of lisdexamfetamine dimesylate, a prodrug of d-amphetamine, for the treatment of attention-deficit/hyperactivity disorder in children and adults.Clin Ther. 2009 Jan;31(1):142-76. doi: 10.1016/j.clinthera.2009.01.015. Clin Ther. 2009. PMID: 19243715 Review.
-
Duration of effect of oral long-acting stimulant medications for ADHD throughout the day.Curr Med Res Opin. 2010 Aug;26(8):1809-25. doi: 10.1185/03007995.2010.488553. Curr Med Res Opin. 2010. PMID: 20491612 Review.
Cited by
-
The Design Features, Quality by Design Approach, Characterization, Therapeutic Applications, and Clinical Considerations of Transdermal Drug Delivery Systems-A Comprehensive Review.Pharmaceuticals (Basel). 2024 Oct 9;17(10):1346. doi: 10.3390/ph17101346. Pharmaceuticals (Basel). 2024. PMID: 39458987 Free PMC article. Review.
-
Recent Advancement of Medical Patch for Transdermal Drug Delivery.Medicina (Kaunas). 2023 Apr 17;59(4):778. doi: 10.3390/medicina59040778. Medicina (Kaunas). 2023. PMID: 37109736 Free PMC article. Review.
-
From Consensus Statement to Pills to Pixels: New Innovations in Attention-Deficit/Hyperactivity Disorder Care.J Child Adolesc Psychopharmacol. 2024 May;34(4):167-182. doi: 10.1089/cap.2024.0022. Epub 2024 Apr 30. J Child Adolesc Psychopharmacol. 2024. PMID: 38686563 Free PMC article. Review.
-
A Review and an Update on Pharmacological Treatment of Children With Attention-Deficit/Hyperactivity Disorder.J Korean Acad Child Adolesc Psychiatry. 2025 Jan 1;36(1):11-17. doi: 10.5765/jkacap.240040. J Korean Acad Child Adolesc Psychiatry. 2025. PMID: 39811027 Free PMC article. No abstract available.
-
Safety of Stimulants Across Patient Populations: A Meta-Analysis.JAMA Netw Open. 2025 May 1;8(5):e259492. doi: 10.1001/jamanetworkopen.2025.9492. JAMA Netw Open. 2025. PMID: 40343695 Free PMC article.
References
-
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Text Revision, 4th ed. Washington, DC: American Psychiatric Association; 2000.
-
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC: American Psychiatric Association; 2013.
-
- Barbaresi WJ, Campbell L, Diekroger EA, Froehlich TE, Liu YH, O'Malley E, Pelham WE Jr., Power TJ, Zinner SH, Chan E: Society for Developmental and Behavioral Pediatrics Clinical Practice Guideline for the assessment and treatment of children and adolescents with complex attention-deficit/hyperactivity disorder. J Dev Behav Pediatr 41:S35–S57, 2020. - PubMed
-
- Caye A, Spadini AV, Karam RG, Grevet EH, Rovaris DL, Bau CH, Rohde LA, Kieling C: Predictors of persistence of ADHD into adulthood: A systematic review of the literature and meta-analysis. Eur Child Adolesc Psychiatry 25:1151–1159, 2016. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
