Adjuvant Capecitabine for Early Breast Cancer: 15-Year Overall Survival Results From a Randomized Trial
- PMID: 35020465
- PMCID: PMC8966968
- DOI: 10.1200/JCO.21.02054
Adjuvant Capecitabine for Early Breast Cancer: 15-Year Overall Survival Results From a Randomized Trial
Abstract
Purpose: Few data are available regarding the influence of adjuvant capecitabine on long-term survival of patients with early breast cancer.
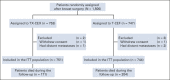
Methods: The Finland Capecitabine Trial (FinXX) is a randomized, open-label, multicenter trial that evaluates integration of capecitabine to an adjuvant chemotherapy regimen containing a taxane and an anthracycline for the treatment of early breast cancer. Between January 27, 2004, and May 29, 2007, 1,500 patients with axillary node-positive or high-risk node-negative early breast cancer were accrued. The patients were randomly allocated to either TX-CEX, consisting of three cycles of docetaxel (T) plus capecitabine (X) followed by three cycles of cyclophosphamide, epirubicin, and capecitabine (CEX, 753 patients), or to T-CEF, consisting of three cycles of docetaxel followed by three cycles of cyclophosphamide, epirubicin, and fluorouracil (CEF, 747 patients). We performed a protocol-scheduled analysis of overall survival on the basis of approximately 15-year follow-up of the patients.
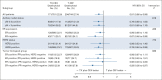
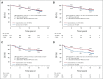
Results: The data collection was locked on December 31, 2020. By this date, the median follow-up time of the patients alive was 15.3 years (interquartile range, 14.5-16.1 years) in the TX-CEX group and 15.4 years (interquartile range, 14.8-16.0 years) in the T-CEF group. Patients assigned to TX-CEX survived longer than those assigned to T-CEF (hazard ratio 0.81; 95% CI, 0.66 to 0.99; P = .037). The 15-year survival rate was 77.6% in the TX-CEX group and 73.3% in the T-CEF group. In exploratory subgroup analyses, patients with estrogen receptor-negative cancer and those with triple-negative cancer treated with TX-CEX tended to live longer than those treated with T-CEF.
Conclusion: Addition of capecitabine to a chemotherapy regimen that contained docetaxel, epirubicin, and cyclophosphamide prolonged the survival of patients with early breast cancer.
Trial registration: ClinicalTrials.gov NCT00114816.
Conflict of interest statement
Figures

References
-
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG) : Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: A patient-level meta-analysis of 37 298 women with early breast cancer in 26 randomised trials. Lancet 393:1440-1452, 2019 - PMC - PubMed
-
- von Minckwitz G, Kummel S, Vogel P, et al. : Neoadjuvant vinorelbine-capecitabine versus docetaxel-doxorubicin-cyclophosphamide in early nonresponsive breast cancer: Phase III randomized GeparTrio trial. J Natl Cancer Inst 100:542-551, 2008 - PubMed
-
- von Minckwitz G, Rezai M, Loibl S, et al. : Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: Phase III GeparQuattro study. J Clin Oncol 28:2015-2023, 2010 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

